Research Article 黑料网
Clinical Impact of the AKT1 rs1130233 SNP in Japanese Gastrointestinal Cancer Patients with Palliative Care
Takuto Morishita1, Asahi Hishida1 *, Yoshinaga Okugawa2, Yuuki Morimoto2, Yumiko Shirai3, Kyoko Okamoto4, Aki Ogawa4, Koji Tanaka2, Ryutaro Nishikawa2, Yuji Toiyama5, Yasuhiro Inoue5, Hiroyuki Sakurai6, Hisashi Urata6, Motoyoshi Tanaka7 and Chikao Miki2
1Department of Preventive Medicine, Nagoya University Graduate School of Medicine, Nagoya 466-8550, Japan
2Departments of Surgery, Iga City General Hospital, Japan
3Department of Nutrition, Iga City General Hospital, Japan
4Department of Nursing, Iga City General Hospital, Japan
5Department of Gastrointestinal and Pediatric Surgery, Division of Reparative Medicine, Institute of LIFe Sciences, Mie University Graduate School of Medicine, Japan
6Department of Hepatobiliary Pancreatic and Transplant Surgery, Mie University Graduate School of Medicine, Japan
7Department of Medical Oncology, Iga City General Hospital, Japan
- *Corresponding Author:
- Asahi Hishida
Department of Preventive Medicine,
Nagoya University Graduate School of Medicine
65 Tsurumai-cho, Showa-ku,
Nagoya 466-8550 Japan
Tel: +81-52-744-2132
Fax: +81-52-744-297
E-mail: a-hishi@med.nagoya-u.ac.jp
Received date: June 12, 2017; Accepted date: June 22, 2017; Published date: June 27, 2017
Citation: Morishita T, Hishida A, Okugawa Y, Morimoto Y, Shirai Y, et al. (2017) Clinical Impact of the AKT1 rs1130233 SNP in Japanese Gastrointestinal Cancer Patients with Palliative Care. J Palliat Care Med 7: 307. doi: 10.4172/2165- 7386.1000307
Copyright: © 2017 Morishita T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Palliative Care & Medicine
Abstract
Objective: Cancer patients often suffer from inflammation, anorexia and the resultant decrease of nutrient intake, followed by weight loss and muscle wasting called “sarcopenia”. Such conditions are known as “cachexia”. In this study, we examined the associations between genetic polymorphisms of AKT1 rs1130233, ICAM1 rs281432, SELP rs6128 and TNSRSF1A rs4149570, which are reportedly associated with cachexia in Caucasians, together with LIF rs929271, in Japanese gastrointestinal cancer patients with palliative care. Methods: The study subjects were 59 patients (37 males and 22 females) with gastrointestinal cancers who visited the outpatient clinic at Iga General Hospital from December 2011 till August 2015. Genotypings for AKT1 rs1130233, ICAM1 rs281432, SELP rs6128, TNSRSF1A rs4149570 and LIF rs929271 were conducted with polymerase chain reaction with confronting two-pair primers (PCR-CTPP) or the Taqman SNP Genotyping assay. Associations of these SNPs with patients’ prognosis as well as weight loss defined as weight loss more than 5 percent during 6 months after the initiation of chemotherapy were evaluated. Results: A significant increase in the risk of 5% weight loss was observed in those with A/G genotype AKT1 rs1130233 polymorphism (AKT1 A/G vs. G/G, adjusted odds ratio [aOR]=7.11; 95%CI: 1.41-35.7), or in those with at least one A allele of AKT1 rs1130233 (AKT1 A/G+A/A vs. G/G, aOR=4.57; 95% CI: 1.14-18.3) when adjusted for age, sex and UICC clinical stage 4. There was no statistically significant correlation of the polymorphisms examined with patients’ survival. Conclusion: The present study revealed that AKT1 rs1130233 A allele may play a key role in the development of cancer cachexia. Given the involvement of AKT1 in the development of cancer as well as in apoptosis, it would be worth studying the roles of this molecule in human cancers further from clinical, epidemiological and biological viewpoints in the near future.
Keywords
Gastrointestinal cancer; Cancer cachexia; Genetic polymorphisms; AKT serine/threonine kinase 1; Weight loss; Apoptosis; Cancer palliative care
Introduction
Cancer is one of the major causes of morbidity and mortality worldwide, and one of the leading causes of deaths in Japan. Gastrointestinal cancers explain the largest part of cancer incidence and mortality in Japan [1], making the palliative care to the patients with these Life-threatening diseases increasingly important in recent years. Cancer patients often suffer from inflammation, anorexia and the resultant decrease of nutrient intake, followed by weight loss and muscle wasting called “sarcopenia”. Such conditions are known as “cachexia”, which worsens patients’ quality of Life, pressing the need for palliative medical care. It is usually induced by the tumor existence and progression, and some genetic polymorphisms are reported as possible causes of cachexia [2,3].
Iga General Hospital in the central of Japan is one of the key hospitals providing palliative care to the corresponding area. There are large amount of patients’ clinical data such as weight, muscle weight of the patients collected and accumulated in the hospital. AKT1 (AKT serine/threonine kinase 1) and SELP (selectin P) polymorphisms are involved in cancer cachexia in pancreatic patients in Caucasians [4]. Polymorphisms of ICAM1 (intercellular adhesion molecule 1) can be a potentially useful biomarker for identifying individuals with higher risk of gastric cancer, predicting disease progression, and guiding individualized treatment [5]. One TNSRSF1A (tumor necrosis factor receptor superfamily 1A) polymorphism is reportedly an important risk marker for T-cell lymphoma via the constitutively elevated TNF-α (tumor necrosis alpha) expression [6]. Recently, LIF (leukemia inhibitory factor) was demonstrated to induce muscle atrophy in the mouse model of colon cancer [7], and the SNP (single nucleotide polymorphism) of LIF rs929271 T/G has been shown to be a susceptibility biomarker capable of predicting implantation efficiency and pregnancy outcomes [8].
In the present study, we examined the associations between genetic polymorphisms of AKT1 rs1130233, ICAM1 rs281432, SELP rs6128 and TNSRSF1A rs4149570, which have been recently suggested to be associated with the risk of cachexia/weight loss in Caucasian cancer patients [4], together with the LIF rs929271, and the risk of cancer cachexia as well as patients’ prognosis in Japanese, leveraging the data from the cancer outpatient clinic of our hospital to find the way for the possible personalized palliative care of gastrointestinal patients based on genetic information.
Patients and Methods
Patients
Analysis of the data from 59 patients (37 males and 22 females) with gastrointestinal cancers who visited the outpatient clinic for cancer chemotherapy and palliative care at Iga General Hospital from December 2011 till August 2015 was conducted. 53 patients underwent surgery and 6 did not. All of them underwent palliative chemotherapy and their body composition (such as total body weight, skeletal muscle weight and water weight) was measured mainly for the analysis of cachexia. All of the patients gave us written informed consent and provided clinical data and blood for the analysis and DNA testing. Patients’ weight loss during 6 months after the initiation of chemotherapy was categorized as weight loss more than 5 percent (denoted as WL). Follow up of the patients’ clinical information/conditions were conducted by checking up their electronic and/or paper medical records.
Genotyping
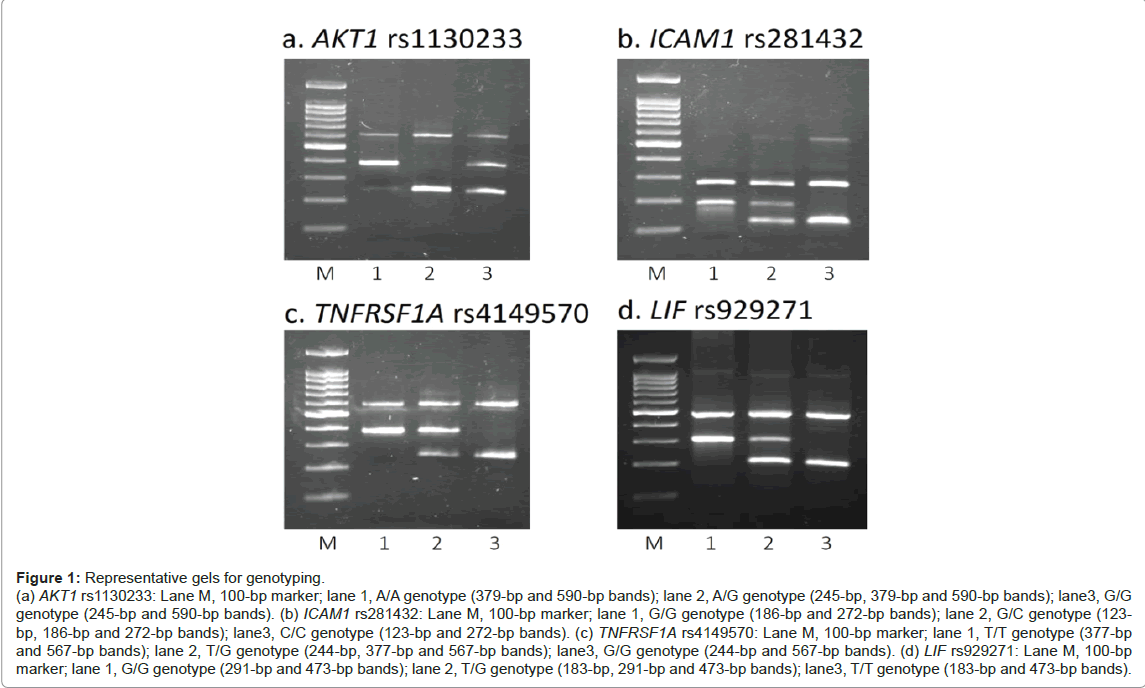
DNA was extracted from the patients’ buffy coat, using the Qiagen Bio Robot EZ1 (Qiagen®, Hilden, Germany). Genotypings of LIF rs929271, AKT1 rs1130233, ICAM1 rs281432, and TNSRSF1A rs4149570 were conducted by polymerase chain reaction with confronting two-pair primers (PCR-CTPP) [9]. The primers used (and the thermal cycler conditions) for each SNP were as follows: F1: GAA CCA GCC CCC TGG AAG, R1: CCT TTC CCT GGT CCC TAC TCA A, F2: AGG GGC AGG GTT GTT CCA and R2: CGG GTG CCT TTC TGT CTT GA (initial denaturation at 95ºC for 10 min, followed by 30 cycles of 95ºC 1 min, 62ºC 1 min and 72ºC 1 min, and the final extension of 72ºC 5 min) for LIF rs929271; F1: CCA CCT GTC CCG GGA A, R1: CGT GTG CTC AGG ACG TGG, F2: ATG CCT GCC CAG GCA G and R2: GTC CTC GGA GAA CAC ACG C (95ºC for 10 min, followed by 30 cycles of 95ºC 1 min, 60ºC 1 min and 72ºC 1 min, and the final extension of 72ºC 5 min) for AKT1 rs1130233; F1: ATA GGG AGT CAT GGA GGG TTT G, R1: CTT TAC CAA ATC CTG GTC ACT GAA, F2: AAA AAA TTG ATT GAT GGG AGG AAG and R2: TAA TCC CTG GCC TGC TCA G (95ºC for 10 min, followed by 30 cycles of 95ºC 1 min, 60ºC 1 min and 72ºC 1 min, and the final extension of 72ºC 5 min) for ICAM1 rs281432; F1: AAT TGG AAA ACA GAT CCA GAC AGT, R1: AGT GAG GCA GTG TTG CAA CAG, F2: CTT TGA GTT TTG GAT TGG ATC AGT and R2: AAT GAA CTT CTC AGA CAC ATA ACT GAA C (95ºC for 10 min, followed by 30 cycles of 95ºC 1 min, 59ºC 1 min and 72ºC 1 min, and the final extension of 72ºC 5 min) for TNSRSF1A rs4149570 (the underline indicates the SNP base). We also genotyped SELP rs6128 by using the Taqman SNP Genotyping Assay (Applied Biosystems Co., Foster City, CA). Representative gels for genotyping are shown in Figure 1.
Figure 1: Representative gels for genotyping. (a) AKT1 rs1130233: Lane M, 100-bp marker; lane 1, A/A genotype (379-bp and 590-bp bands); lane 2, A/G genotype (245-bp, 379-bp and 590-bp bands); lane3, G/G genotype (245-bp and 590-bp bands). (b) ICAM1 rs281432: Lane M, 100-bp marker; lane 1, G/G genotype (186-bp and 272-bp bands); lane 2, G/C genotype (123- bp, 186-bp and 272-bp bands); lane3, C/C genotype (123-bp and 272-bp bands). (c) TNFRSF1A rs4149570: Lane M, 100-bp marker; lane 1, T/T genotype (377-bp and 567-bp bands); lane 2, T/G genotype (244-bp, 377-bp and 567-bp bands); lane3, G/G genotype (244-bp and 567-bp bands). (d) LIF rs929271: Lane M, 100-bp marker; lane 1, G/G genotype (291-bp and 473-bp bands); lane 2, T/G genotype (183-bp, 291-bp and 473-bp bands); lane3, T/T genotype (183-bp and 473-bp bands).
Statistical analysis
To evaluate survival time, Kaplan-Meier Curve, the logrank test, the Wilcoxon test and the Cox proportional hazard model were used. In addition, we analyzed the effect of genotype on patients’ body weight by using the logistic regression model. The statistical software, STATA ver.13 (STATA Corp, TX), was used for analysis. This study was approved by the Institutional Review Board of Nagoya University Graduate School of Medicine (Approval no.2013-0220-08)
Results
Characteristics of patients are shown in Table 1. Table 2 shows the risk of weight loss (WL) with the crude OR (odds ratio), aOR-1 (sexand age-adjusted odds ratio) and aOR-2 (sex- age- and clinical stage 4-adjusted odds ratio) by genotypes. A significant increase in the risk of WL in those with the A/G genotype of AKT1 rs1130233 polymorphism (AKT1 A/G vs. G/G, aOR-2=7.11; 95%CI: 1.41-35.7), or in those with at least one A allele of AKT1 rs1130233 (AKT1 A/G+A/A vs. G/G, aOR- 2=4.57; 95% CI: 1.14-18.3) was observed. This association remained significant when the patients were restricted to those with colorectal cancers (AKT1 A/G+A/A vs. G/G, aOR-2=6.59; 95% CI: 1.09-39.7) (Table 3). No other polymorphisms showed statistical significance.
| Variables | Values |
|---|---|
| Age [y (sd)] | 68.1 (12.2) |
| Sex [n (%)] | |
| Male | 37 (62.7) |
| Female | 22 (37.3) |
| Cancer Type [n (%)] | |
| Esophageal | 2 (3.4) |
| Stomach | 11 (18.6) |
| Colorectal | 40 (67.8) |
| Pancreatic | 5 (8.5) |
| Billiary | 1 (1.7) |
| UICC Stage [n (%)] | |
| I | 5 (8.5) |
| II | 10 (16.9) |
| III | 15 (25.4) |
| IV | 29 (49.2) |
| Genotype Frequency[n (%)] | |
| SELP rs6128 | |
| A/A | 14 (23.73) |
| A/G | 31 (52.54) |
| G/G | 14 (23.73) |
| AKT1rs1130233 | |
| A/A | 12 (20.34) |
| A/G | 29 (49.15) |
| G/G | 18 (30.51) |
| ICAM1rs281432 | |
| C/C | 28 (50.00) |
| G/C | 22 (39.29) |
| G/G | 6 (10.71) |
| TNFRSF1Ars4149570 | |
| G/G | 22 (37.29) |
| T/G | 28 (47.46) |
| T/T | 9 (15.25) |
| LIFrs929271 | |
| G/G | 8 (13.56) |
| G/T | 32 (54.24) |
| T/T | 19 (32.20) |
Table 1: Characteristics of the study subjects.
| SNP & genotype | WL (+) | WL(-) | crude OR | aOR-1 | aOR-2 |
|---|---|---|---|---|---|
| SELP rs6128 | |||||
| A/A | 8 | 3 | 1 | 1 | 1 |
| A/G | 15 | 12 | 0.46 (0.10-2.16) | 0.45 (0.09-2.16) | 0.71 (0.13-3.80) |
| G/G | 9 | 4 | 0.84 (0.14-4.97) | 0.82 (0.13-5.03) | 1.38 (0.19-9.91) |
| AKT1rs1130233 | |||||
| G/G | 6 | 9 | 1 | 1 | 1 |
| A/G | 20 | 6 | 5 (1.26-19.8) | 6.20 (1.42-27.0) | 7.11 (1.41-35.7) |
| A/A | 6 | 4 | 2.25 (0.43-11.5) | 2.23 (0.42-11.8) | 2.23 (0.39-12.6) |
| A/G+A/A | 26 | 10 | 3.9 (1.10-13.8) | 4.37 (1.17-16.2) | 4.57 (1.14-18.3) |
| ICAM1rs281432 | |||||
| C/C | 13 | 10 | 1 | 1 | 1 |
| G/C | 15 | 5 | 2.2 (0.61-7.88) | 2.15 (0.59-7.80) | 2.41 (0.62-9.32) |
| G/G | 2 | 3 | 0.48 (0.06-3.43) | 0.50 (0.07-3.60) | 0.76 (0.09-5.87) |
| TNFRSF1A rs4149570 | |||||
| G/G | 9 | 9 | 1 | 1 | 1 |
| T/G | 19 | 7 | 2.71 (0.76-9.63) | 2.77 (0.70-10.9) | 2.55 (0.60-10.7) |
| T/T | 4 | 3 | 1.33 (0.22-7.74) | 1.27 (0.20-8.02) | 1.15 (0.16-7.97) |
| LIFrs929271 | |||||
| T/T | 10 | 5 | 1 | 1 | 1 |
| G/T | 18 | 11 | 0.81 (0.68-5.85) | 0.82 (0.21-3.08) | 0.87 (0.21-3.50) |
| G/G | 4 | 3 | 0.66 (0.10-4.20) | 0.69 (0.10-4.46) | 0.72 (0.10-5.01) |
| WL (+) indicates the number of subjects with weight loss more than 5%; WL (-) indicates those with no weight loss. SNP:Single Nucleotide Polymorphism; OR:Odds Ratio (95% confidence interval in the parenthesis); aOR:Adjusted Odds Ratio (adjusted for age and sex); aOR-2:Adjusted Odds Ratio(adjusted for age, sex and UICC clinical stage 4) |
|||||
Table 2: Risk of weight loss by genotypes.
| SNP and genotype | WL (+) | WL(-) | crude OR | aOR-1 | aOR-2 |
|---|---|---|---|---|---|
| AKT1rs1130233 | |||||
| G/G | 4 | 8 | 1 | 1 | 1 |
| A/G | 11 | 4 | 5.49 (1.04-28.8) | 5.45 (0.95-30.9) | 6.47 (0.89-46.9) |
| A/A | 4 | 2 | 4 (0.50-31.9) | 4.13 (0.49-34.3) | 6.79 (0.67-68.2) |
| A/G+A/A | 15 | 6 | 5.0 (1.08-23.0) | 4.97 (1.02-24.1) | 6.59 (1.09-39.7) |
| WL (+) indicates the number of subjects with weight loss more than 5%; WL (-) indicates those with no weight loss. SNP:Single Nucleotide Polymorphism; OR:Odds Ratio (95% confidence interval in the parenthesis); aOR:Adjusted Odds Ratio (adjusted for age and sex); aOR-2:Adjusted Odds Ratio (adjusted for age, sex and UICC clinical stage4). | |||||
Table 3: Risk of weight loss by AKT1 genotypes in colorectal cancer patients.
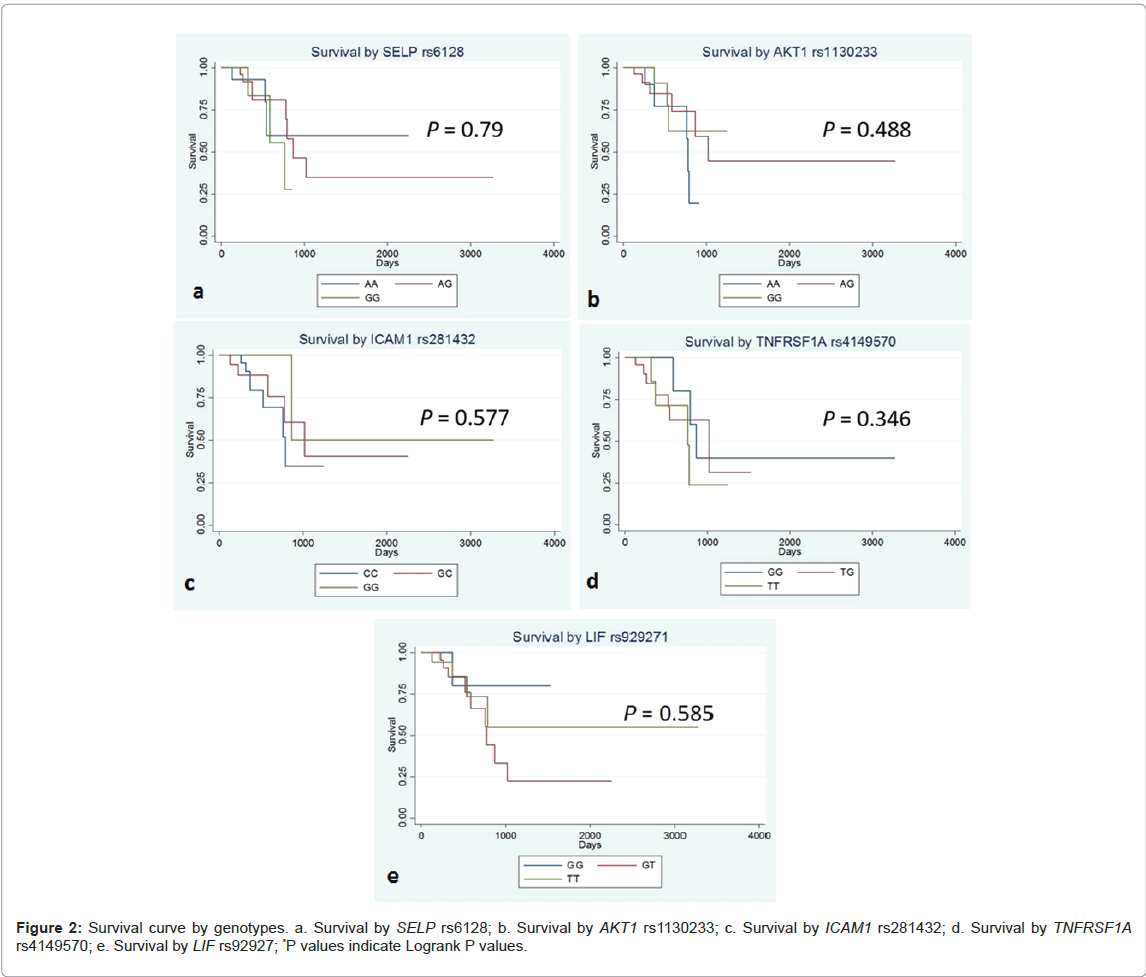
Table 4 shows logrank P value and crude HR (hazard ratio) and the aHR (adjusted hazard ratio) for patients’ survival. Kaplan-Meier Curves for the patients’ survival by genotypes are shown in Figure 2. There was no statistically significant association of these 5 SNPs with patients’ survival.
| SNP | Comparison groups | LogrankP | Model | Crude HR | aHR-1 | aHR-2 |
|---|---|---|---|---|---|---|
| SELP rs6128 | all 3 genotypes | 0.79 | additive | 1.30 (0.56-3.02) | 1.39 (0.59-3.26) | 1.24 (0.51-3.00) |
| vt.hetero+vt. homo vs. wt. homo | 0.727 | dominant | 1.25 (0.34-4.52) | 1.44 (0.37-5.49) | 1.25 (0.33-4.75) | |
| vt. homo vs. others | 0.508 | recessive | 1.55 (0.41-5.84) | 1.68 (0.42-6.65) | 1.48 (0.32-6.75) | |
| AKT1rs1130233 | all 3 genotypes | 0.488 | additive | 1.43 (0.65-3.14) | 1.39 (0.52-3.72) | 1.10 (0.42-2.88) |
| vt.hetero+vt. homo vs. wt. homo | 0.76 | dominant | 1.22 (0.33-4.55) | 0.95 (0.21-4.16) | 0.80 (0.185-3.50) | |
| vt. homo vs. others | 0.232 | recessive | 1.96 (0.63-6.06) | 2.05 (0.53-7.93) | 1.52 (0.38-6.07) | |
| ICAM1rs281432 | all 3 genotypes | 0.461 | additive | 0.58 (0.24-1.39) | 0.43 (0.15-1.22) | 0.61 (0.19-1.96) |
| vt.hetero+vt. homo vs. wt. homo | 0.243 | dominant | 0.52 (0.17-1.58) | 0.35 (0.09-1.27) | 0.48 (0.12-1.85) | |
| vt. homo vs. others | 0.43 | recessive | 0.04 (0.05-3.47) | 0.41 (0.05-3.28) | 1.29 (0.12-13.7) | |
| TNFRSF1Ars4149570 | all 3 genotypes | 0.346 | additive | 1.66 (0.82-3.38) | 2.06 (0.92-4.61) | 1.85 (0.78-4.37) |
| vt.hetero+vt. homo vs. wt. homo | 0.173 | dominant | 2.37 (0.65-8.52) | 2.49 (0.688-9.07) | 1.82 (0.48-6.80) | |
| vt. homo vs. others | 0.318 | recessive | 1.79 (0.55-5.76) | 2.99 (0.80-11.1) | 3.11 (0.78-12.3) | |
| LIFrs929271 | all 3 genotypes | 0.585 | additive | 1.00 (0.45-2.22) | 1.04 (0.47-2.31) | 0.97 (0.43-2.17) |
| vt.hetero+vt. homo vs. wt. homo | 0.602 | dominant | 1.36 (0.42-4.38) | 1.56 (0.47-5.17) | 1.46 (0.43-4.86) | |
| vt. homo vs. others | 0.479 | recessive | 0.48 (0.06-3.74) | 0.43 (0.05-3.50) | 0.37 (0.04-3.03) | |
| SNP: Single Nucleotide Polymorphism; HR: Hazard Ratio; aHR: Adjusted Hazard Ratio (aHR-1, adjusted for sex and age; aHR-2, adjusted for age, sex and UICC clinical stage 4). | ||||||
Table 4: Patient prognosis by genotypes.
Discussion
There are a considerable number of studies on the relationship between SNPs and cancer risk or prognosis worldwide, but studies on the associations between SNPs and cachexia still remain scarce. One previous study suggested that SELP and AKT1 polymorphisms may play roles in the risk of cachexia and death in pancreatic ductal adenocarcinoma patients (PDAC) in Caucasians [4]. In the present study, SELP and AKT1 SNPs were analyzed and significant association was found on AKT1, where the risk of 5% weight loss in patients with the AKT1 A allele significantly increased. This finding was in the same trend as that reported by Avan et al. [4], suggesting that the AKT1 A allele may play a key role in the development of cancer cachexia. There were no significant effects of the SNPs examined on patients’ survival, presumably due to the relatively smaller sample size of the present study. AKT1 responds to various stimuli such as hormones and growth factors, and it is involved in glucose metabolism, cell growth, and angiogenesis [10,11]. In cancer patients, the expression of AKT1 increase, as cancer cells grow. AKT1 is shown to exert its effects through various mediators, such as protein kinases and phosphatases, survival factors, regulators of protein synthesis, and so on, thereby play key roles in the development of cancer, cardiovascular diseases or metabolic diseases [12]. In human cancer cachexia, reduced activity of AKT1 in those with AKT1 rs1130233 G/A+A/A genotypes is considered to favor the induction of apoptosis, resulting in the higher risk of muscle atrophy and cachexia[4]. In the body of cachexia patients, production of inflammatory cytokine is induced, leading to the breakdown of fat and muscle. Given these biological facts reported, this reduced AKT1 activity due to this AKT1 SNP may lead to the development of severer weight loss in gastrointestinal cancer patients who suffer from tumorinduced inflammation. The allele frequencies of AKT1 rs1130233 differ by race, with the A allele frequency of 0.300 in Caucasians, 0.051 in Africans and 0.575 in East Asians (consisting of Japanese and Chinese) according to dbSNP (http://www.ncbi.nlm.nih.gov/projects/SNP), suggesting the possible existence of population specific susceptibility to cancer cachexia. The roles of AKT1 in human cancer cachexia in various races/ethnicities need to be further verified in the near future.
The other functional SNPs in SELP, ICAM, TNSRSF1A and LIF genes did not show any significance, suggesting the limited roles of these SNPs in cachexia of gastrointestinal cancer patients. The data in this study was gathered once or twice a week, making it a minute and detailed data. The number of subjects in the present study was 59, the statistical power of which was more than 50% for an OR of 4 or 0.25 with a two-sided α-error of 0.05, when a genotype frequency among the controls was between 40% and 60% with the allocation ratio of 1.5:1 - 0.67:1 by case/control status. With regard to the survival analysis, the statistical power is more than 90% for an HR of 3 or 0.33 with a two-sided α-error of 0.05, when a genotype frequency was between 33% and 67%, while it is more than 55% for an HR of 2 or 0.5 under the same conditions. Although the present study could not reproduce the previously reported association of AKT1 SNP with risk of weight loss in pancreatic cancer patients, it revealed the novel association of the corresponding AKT1 rs1130233 SNP with weight loss in colorectal cancer patients as well as gastrointestinal cancer patients as a whole, suggesting the possible feasibility and usefulness of examining this AKT1 SNP in gastrointestinal cancer patients under palliative care.
Conclusions
The present study suggested the possibly important role of AKT1 in the development of cachexia in gastrointestinal cancer patients. Further investigation of the role of this AKT1 SNP in the context of gastrointestinal cancer palliative care, as well as those with regard to the biological roles of AKT1 in human cancer cachexia would help better understand the potential roles of this molecule in the near future.
Acknowledgements
The authors are also grateful to Ms. Sachiko Momokita, Ms. Yoko Mitsuda and Ms. Keiko Shibata for their technical assistance. This study was supported in part by Grants-in-Aid for Scientiï¬聛c Research from the Japanese Ministry of Education, Culture, Sports, Science and Technology, JSPS KAKENHI Grant Number JP (No. 25460745).
Conflict-of-interest statement
The authors declare that they have no financial conflicts of interest to disclose.
References
- Katanoda K, Hori M, Matsuda T, Shibata A, Nishino Y, et al. (2015)
- Fearon KC, Glass DJ, Guttridge DC (2012)
- Tan BH, Ross JA, Kaasa S, Skorpen F, Fearon KC (2011)
- Avan A, Avan A, Le Large TY, Mambrini A, Funel N, et al. (2014)
- Tian MM, Sun Y, Li ZW, Wu Y, Zhao AL, et al. (2012)
- Zhai K, Chang J, Wu C, Lu N, Huang LM, et al. (2012)
- Seto DN, Kandarian SC, Jackman RW (2015)
- Oliveira JB, Vagnini LD, Petersen CG, Renzi A, Oliveira-Pelegrin GR, et al. (2016)
- Hamajima N, Saito T, Matsuo K, Kozaki K, Takahashi T, et al. (2000)
- Coffer PJ, Jin J, Woodgett JR (1998)
- Anderson KE, Coadwell J, Stephens LR, Hawkins PT (1998)
- Hers I, Vincent EE, Tavaré JM (2011)
Share This Article
Relevant Topics
- Caregiver Support Programs
- End of Life Care
- End-of-Life Communication
- Ethics in Palliative
- Euthanasia
- Family Caregiver
- Geriatric Care
- Holistic Care
- Home Care
- Hospice Care
- Hospice Palliative Care
- Old Age Care
- Palliative Care
- Palliative Care and Euthanasia
- Palliative Care Drugs
- Palliative Care in Oncology
- Palliative Care Medications
- Palliative Care Nursing
- Palliative Medicare
- Palliative Neurology
- Palliative Oncology
- Palliative Psychology
- Palliative Sedation
- Palliative Surgery
- Palliative Treatment
- Pediatric Palliative Care
- Volunteer Palliative Care
Recommended Journals
- Journal of Cardiac and Pulmonary Rehabilitation
- Journal of Community & Public Health Nursing
- Journal of Community & Public Health Nursing
- Journal of Health Care and Prevention
- Journal of Health Care and Prevention
- Journal of Paediatric Medicine & Surgery
- Journal of Paediatric Medicine & Surgery
- Journal of Pain & Relief
- Palliative Care & Medicine
- Journal of Pain & Relief
- Journal of Pediatric Neurological Disorders
- Neonatal and Pediatric Medicine
- Neonatal and Pediatric Medicine
- Neuroscience and Psychiatry: 黑料网
- OMICS Journal of Radiology
- The Psychiatrist: Clinical and Therapeutic Journal
Article Tools
Article Usage
- Total views: 3059
- [From(publication date):
July-2017 - Nov 25, 2024] - Breakdown by view type
- HTML page views : 2348
- PDF downloads : 711