Our Group organises 3000+ Global Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ 黑料网 Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
黑料网 Journals gaining more Readers and Citations
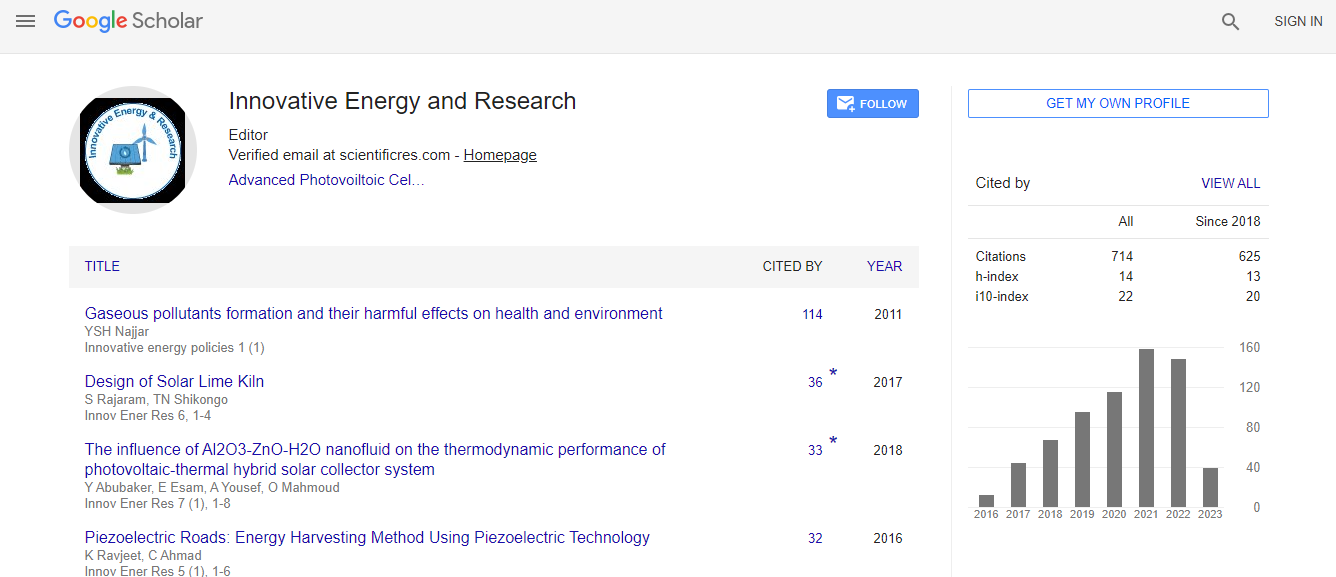
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
Citations : 712
Indexed In
- Google Scholar
- Open J Gate
- Genamics JournalSeek
- RefSeek
- Hamdard University
- EBSCO A-Z
- Publons
- Euro Pub
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
High surface area carbon supported PtPdSn for methyl formate, methanol and formic acid oxidation
21st International Conference on Advanced Energy Materials and Research
Svetlana Lyssenko, Hanan Teller and Alex Schechter
Ariel University, Israel
Posters & Accepted Abstracts: Innov Ener Res
Abstract
Proton exchange membrane fuel cells have been investigated as an alternative power source for portable electronic devices and electric vehicles utilizing mainly hydrogen or small organic molecules (SOM) as fuel source and oxygen form air as an oxidant, at the anode and cathode respectively. The advantages of this type of fuel cells are high energy conversion efficiency directly to electricity and low operation temperatures. The slow kinetics of fuel oxidation (i.e. methanol, ethanol etc.) is the major obstacle for the commercialization of fuel cell technology based on these fuels, in addition to the high price of Pt and Pd based catalyst typically used in the anode. An addition of oxophilic second or third atom, such as Ru, Sn, Ni, etc., to Pt or Pd improves the catalytic activity and tolerance towards the presence of poisoning species during the oxidation of SOM. Carbon materials are commonly used as support materials due to their good electronic conductivity and high specific surface area that influences the properties of the catalyst, such as, charge transfer and improved dispersity of the catalyst particles. This leads to the increase in the utilization of the precious metal constituent. Ternary catalyst (Pt3Pd3Sn2) supported on different carbon materials with different surface area (Vulcan CX72, Multi Walled Carbon Nanotubes and Black Pearl 2000 carbon) have been studied for electro-oxidation of methanol, formic acid and methyl formate. The electrochemical result shows that the MWCNT supported PtPdSn catalyst exhibits higher electro-oxidation current of methanol, methyl formate and formic acid compared to PtPdSn/XC72 and PtPdSn/BP2000.Biography
Svetlana Lyssenko is a Master student in Fuel cell and Electrochemistry group, Ariel University, Israel under the supervision of Prof Alex Schechter. The key findings of her research work are to study the high surface area carbon supported platinum-based anode catalysts for methanol, methyl formate and formic acid electrooxidation for fuel cell applications.
E-mail: atevs92@hotmail.com