Our Group organises 3000+ Global Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ 黑料网 Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
黑料网 Journals gaining more Readers and Citations
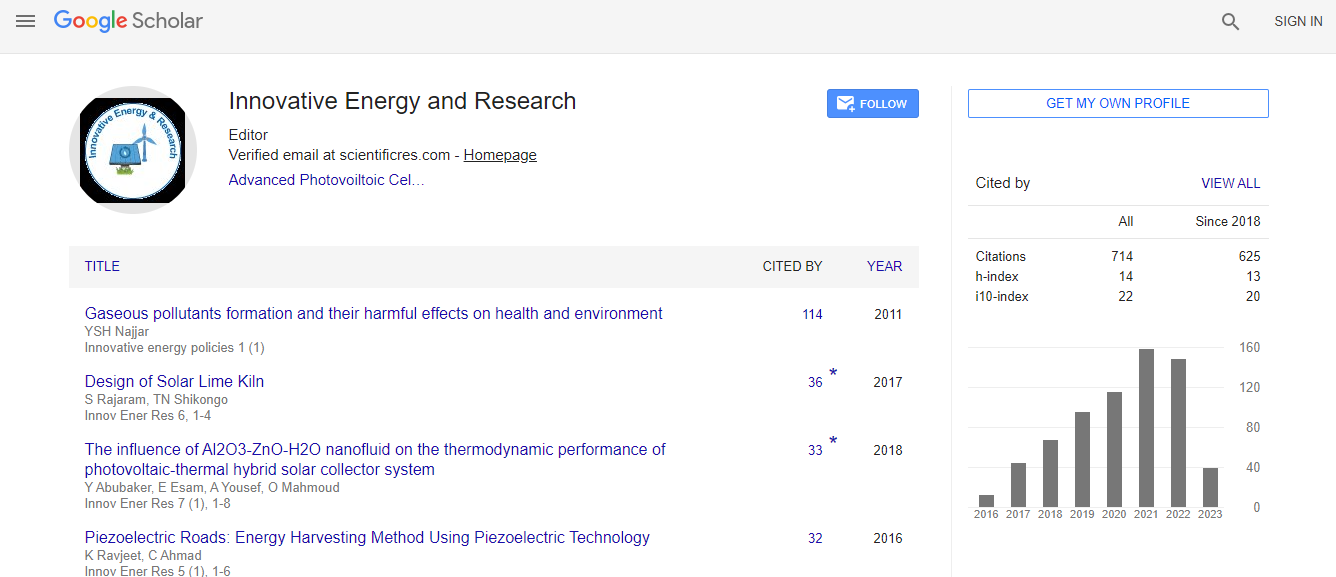
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
Citations : 712
Indexed In
- Google Scholar
- Open J Gate
- Genamics JournalSeek
- RefSeek
- Hamdard University
- EBSCO A-Z
- Publons
- Euro Pub
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
Support less Ag-Rh bimetallic nanostructures as efficient cathodic electro catalyst for di oxygen reduction in alkaline fuel cells
20th International Conference on Advanced Energy Materials and Research
Il Shik Moon, B Narayanamoorthy and S Balaji
Sunchon National University, South KoreaSri Chandrasekharendra Saraswathi Viswa Mahavidyalaya, India
Posters & Accepted Abstracts: Innov Ener Res
DOI:
Abstract
The non-platinum d-block elements can be employed as ORR electrocatalysts due to their catalytic activity which help us to reduce or replace the usage of expensive precious metal catalysts in alkaline fuel cells. A number of cathode electrocatalysts are available such as non-platinum metals (Ag, Au, Pd, etc.) and the non-noble metals (Fe, Co, Ni, Cu, etc.) but they either suffer from low activity or poor stability. Among the noble metal electrocatalysts, Ag is relatively inexpensive (ca. 1% the price of Pt), abundant and reasonably active catalyst with moderate stability and therefore, Ag is an attractive choice for enhancing the kinetics of oxygen reduction in alkaline medium. The redox potential of Rh3+ to Rh is 0.76 V whereas the redox potential of Ag+ to Ag is 0.8 V. The electrochemical activity of pure Rh is very poor but it could provide the additional stability when mixed with other catalyst materials. Hence, formation of Ag-Rh nanostructures may provide to be useful approach for improving the stability in a cost effective way. Silver-rhodium (Ag-Rh) nanostructured electro catalysts were synthesized by one step chemical reduction method and used to catalyze the oxygen reduction reaction (ORR) in an alkaline medium. The crystalline nature was ascertained by x-ray diffraction (XRD), elemental composition was estimated by energy dispersive spectroscopy and morphology was confirmed high resolution transmission electron microscope. The electrochemical properties were studied by cyclic and linear scan voltammetry techniques under hydrodynamic conditions. The supportless Ag-Rh catalyst exhibited a good catalytic activity for ORR and the quantified values in terms of higher mass and intrinsic activities were determined to be 951.7 mA/mg and 1.45 mA/cm2 respectively. Accelerated durability test revealed that the catalyst could withstand nearly 7000 potential cycles with 5% increment in limited current density while retaining nearly 70% of its initial electrochemically active surface area. This study indicates the superior activity and stability of Ag-Rh (compared to Ag-Rh/VC) undoubtedly places it as one among the promising electrocatalysts for ORR in alkaline medium. Recent Publications: 1. Slanac D A, Hardin W G, Johnston K P, Stevenson K J (2012) Atomic ensemble and electronic effects in Ag-rich Ag- Pd nanoalloy catalysts for oxygen reduction in alkaline media, Journal of American Chemical Society 134:9812-9819. 2. Bin F, Jin L, Peter N, Rameshwori L, Derrick M, Bridgid W, Xiang H and Chuan-Jian Z (2009) Nanostructured PtVFe catalysts: Electrocatalytic performance in proton exchange membrane fuel cells, Electrochemistry Communications 11:1139-1141. 3. Nguyen S T, Law H M, Nguyen H T, Kristian N, Wang S, Chan S H, Wang X (2009) Enhancement effect of Ag for Pd/C towards the ethanol electro-oxidation in alkaline media, Applied Catalysis B, 91:507-515. 4. Narayanamoorthy B, Balaji S, Sita C, Pasupathi S, Eswaramoorthy M, Il-Shik Moon (2016) Enhanced Intrinsic Activity and Stability of Au-Rh Bimetallic Nanostructures as a Supportless Cathode Electrocatalyst for Oxygen Reduction in Alkaline Fuel Cells, ACS Sustainable Chemistry and Engineering, 4:6480-6490. 5. Yasmin S, Ahmed M S, Jeon S (2016) A noble silver nanoflower on nitrogen doped carbon nanotube for enhanced oxygen reduction reaction, International Journal of Hydrogen Energy, 42:1075-1084.Biography
Il Shik Moon is working as full Professor at Sunchon National University, Suncheon, South Korea. He has over 18 years of experience on the electrochemically assisted removal of liquid and air pollutants at electro-scrubbing process and its design development sector. His credentials include a Master of Engineering (ME) in Chemical Engineering, and Bachelor of Engineering (BEng) in Chemical Engineering. His expertise includes desalination using DCMD (direct contact membrane desalination) with modelling. Currently, he has colloborate work at Crandfield University, UK, for CO2 removal by electro-scrubbing process.
E-mail: ismoon@sunchon.ac.kr