Our Group organises 3000+ Global Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ 黑料网 Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
黑料网 Journals gaining more Readers and Citations
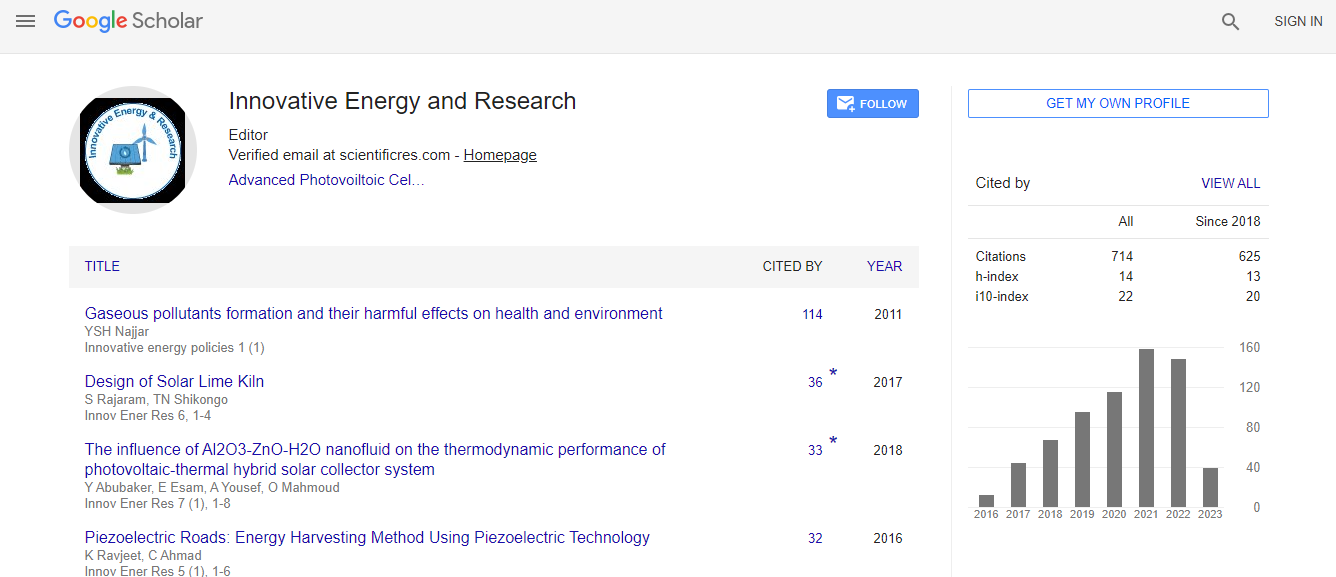
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
Citations : 712
Indexed In
- Google Scholar
- Open J Gate
- Genamics JournalSeek
- RefSeek
- Hamdard University
- EBSCO A-Z
- Publons
- Euro Pub
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
Water contained ionic liquid medium for V(III)(acac)3 reduction: A paired electrolysis and application studies
20th International Conference on Advanced Energy Materials and Research
Il Shik Moon and G Muthuraman
Sunchon National University, South Korea
Posters & Accepted Abstracts: Innov Ener Res
DOI:
Abstract
Statment of the Problem: The green solvent nature of the ionic liquids have potential application in many fields espacialy battery, sensor, electro-organic synthesis due to non-volatile and having wide electrochemical potential window. However, the generation of an electro-active species by paired-electrolysis is a difficult task. Inorder to harvest the high value of the ionic liquid, herein, water content effect was investigated to reduce the V(III)(acetylacetone)3 and its application as reductant. Initial water content analysis with a 1-butyl-3 methyl imidazolium trifluoromethane sulfonate [BMIM CF3SO3] ionic liquid revealed a minimum cell potential of 6 V at 18 M water. Methodology: A Nafion 324 membrane divided plate and frame electrolytic cell was adopted for the paired electrolysis experiments and the resutls obtined by a constant applied current method. Findings: Along with V(III)(acetylacetanone)3, other two compounds Ce(III)(SO4)2 and [Co(II)(CN)5]3-, were tested in the water contained ionic liquid medium. The potentiometric titration with H2O2 enabled reuse of the spent ionic liquid after mediator quantification. The electrolytic reduction of V(III)(acetylacetonate) metal complex in 18 M water-containing BMIM CF3SO3 under optimized conditions revealed 65% of V(II)(acetylacetonate) formation. The applicability was checked by using an organic compound dichloromethane, where found a well-defined change in the concentration of V(III)(acetylacetonate) from 18% to 6% upon the addition of 20 mM dichloromethane demonstrated the that dichloromethane reduction follows the mediated electrochemical reaction (MER). Conclusions & Signifigans: The developed system allows the use of galvanostatic mode to generate a electron active species in an ionic liquid medium. Recent Publications: 1. Alvarez Guerra M, Albo J, Alvarez Guerra E and Irabien A (2015) Ionic liquids in the electrochemical valorisation of CO2. Energy & Environmental Science 8(9):2574-2599. 2. Reddy P N, Padmaja P, Subba Reddy B V and Rambabu G (2015) Ionic liquid/water mixture promoted organic transformations. RSC Advances 5(63):51035-51054. 3. Francke R and Little R D (2014) Redox catalysis in organic electrosynthesis: basic principles and recent developments. Chemical Society Reviews 43(8):2492-2521. 4. Bornemann S and Handy S T (2011) Synthetic organic electrochemistry in ionic liquids: the viscosity question. Molecules 16(7):5963. 5. Balaji S, Kannan K and Moon I S (2015) The electrochemical oxidation of toluene catalysed by Co(II) in N-butyl-Nmethylpyrrolidinium bis(trifluoromethylsulfonyl)imide. Physical Chemistry Chemical Physics 17:30983-30987.Biography
I S Moon is working as full Professor at Sunchon National University, Suncheon, South Korea. He has over 18 years of experience on the electrochemically assisted removal of liquid and air pollutants at electro-scrubbing process and its design development sector. His credentials include a Master of Engineering (ME) in Chemical Engineering, and Bachelor of Engineering (BEng) in Chemical Engineering. His expertise includes desalination using DCMD (direct contact membrane desalination) with modelling. Currently, he has colloborate work at Crandfield University, UK, for CO2 removal by electro-scrubbing process.
E-mail: ismoon@sunchon.ac.kr