Review Article 黑料网
Antibodies to a Brain-Type Creatine Kinase: Biomarker of Cancer-Related Paraneoplastic Neurological Syndromes
Syuichi Tetsuka*
Department of Neurology, Hospital of International University of Health and Welfare, Japan
- *Corresponding Author:
- Syuichi Tetsuka
Department of Obstetrics & Gynecology
Hospital of International University of Health and Welfare
537-3, Iguchi,Nasushiobara
Tochigi, 329-2763
Japan
Tel: +81-287-37-2221
Fax: +81-287-39-3001
E-mail: syuichi@jichi.ac.jp
Received date: May 07, 2016; Accepted date: July 05, 2016; Published date: July 11, 2016
Citation: Tetsuka (2016) Antibodies to a Brain-Type Creatine Kinase: Biomarker of Cancer-Related Paraneoplastic Neurological Syndromes. J Clin Exp Neuroimmunol 1:106. doi:10.4172/jceni.1000106
Copyright: © 2016 Tetsuka S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Clinical & Experimental Neuroimmunology
Abstract
The primary pathological mechanism for paraneoplastic neurological syndromes is believed to be a form of onconeural immunity where the cancer causes a cross-immune reaction with the neurons. In a previous study, using a proteomic approach, we detected an anti-brain-type creatine kinase antibody that was associated with paraneoplastic cerebellar degeneration. Using immunohistochemistry, we showed that this antibody reacted with both mouse and human cerebellar neurons, as well as bladder cancer cells, small cell lung cancer cells, and Hodgkin’s lymphoma tissues. In the current study, this antibody was detected in sera from two paraneoplastic cerebellar degeneration patients with small cell lung cancer and one lymphoma patient in whom the sensory-motor neuropathy manifested as paraneoplastic neurological syndrome. In total, we detected five patients with paraneoplastic neurological syndrome. In the previous studies, as far as we could find, five out of six paraneoplastic neurological syndrome patients who tested negative for well-characterized onconeural antibodies were positive for anti-brain-type creatine kinase antibodies. It is high frequency. Taking into account these findings, brain-type creatine kinase may be a good candidate as an onconeural antigen for many cancers. Paraneoplastic neurological syndromes associated with anti-brain-type creatine kinase autoantibody may be the cause of some predominant cancers, which is similar in other well-characterized onconeural antibodies. However, it is necessary to perform further epidemiological analysis to confirm the presence of anti-brain-type creatine kinase antibody before it can be considered as a useful marker of PNS in the setting.
Keywords
Autoimmune disease; Cancer; Creatine kinase braintype; Intracellular antigen; Paraneoplastic neurological syndromes
Introduction
Paraneoplastic neurological syndrome (PNS) can have an undue influence on any part of the nervous system. There have been cases wherein such syndromes occur as the first sign of a tumor or lead to its detection. Commonly, they emerge during the progression of cancer. Survival is usually influenced by the progression of cancer, but PNS can cause severe neurologic disability and may be fatal. PNSs are relatively rare and occur due to the remote effect of a tumor; they are metastases that are not directly caused by mass lesions. The primary pathological mechanism for PNS is considered to be a form of onconeural immunology wherein a cancer causes a cross-immune reaction with neurons. An immune response that targets these proteins can then cross-react with neurons that express the same proteins. One of the most common forms of PNS is paraneoplastic cerebellar degeneration (PCD).
The subject of this study was a PCD patient with urothelial carcinomas of the urinary bladder who was negative for the wellcharacterized PNS-related onconeural antibodies. In the previous study, a new PCD-related onconeural antibody was identified, and we were able to recognize both cerebellar neurons and cancer tissues from the patient and utilized a proteomic approach using mass spectrometry after two-dimensional and Western blotting. (CKB) was identified as a new autoantigen in the serum and cerebrospinal fluid of the patient [1]. indicated that anti-CKB antibodies reacted with both cerebellar neurons and urothelial carcinomas of the urinary bladder tissues. Immunohistochemical analysis of a bladder cancer tissue array showed high incidence of CKB expression, although an anti-CKB antibody was not recognized in sera from over 30 blood donors, including bladder cancer patients without PCD. This result indicated that anti-CKB antibody is onconeurally immune to the onset of PCD. Anti-CKB antibodies were also detected in sera from three other PCD patients. The study showed that anti-CKB antibody may be added to the list of PCD-related autoantibodies and may be useful for the diagnosis of PCD or PNS. Moreover, to verify the new onconeural antibody, further investigation was conducted on whether the expression of this autoantigen is presented in both human cerebellum and cancer tissues (small cell lung cancer and Hodgkin’s lymphoma). This autoantigen expression was detected in both the abovementioned specimens by immunohistochemistry analysis. In addition, this antibody was also detected in the serum of the patient with sensorymotor neuropathy that manifested as PNS. This antibody was identified in five PNS patients, and this autoantigen expression was recognized in the human cerebellum and cancer [2]. These results demonstrated that anti-CKB antibody might be a novel antibody with regard to PNS. In this review, we summarize two previous studies [1,2] and describe some important factors of anti-CKB antibody that has the potential to be a novel onconeural antibody associated with PNS.
Incidence
Although the exact incidence of PNS among those diagnosed with cancer is still uncertain, it is estimated that PNS affects 1%-25% of cancer patients [3]. This incidence is likely to increase, because cancer patients are living longer, and the diagnostic methods for PNS may improve in the future, proceeding PNS studies like ours. One of the most common types of PNS is PCD [4], which is initially characterized by sub-acute cerebellar ataxia, and patients ultimately develop severe sequela due to the death of Purkinje cells in the cerebellum. It has been reported that only approximately 36% of PCD patients are positive for the known onconeural antibodies associated with PCD [5]. To date, these estimates come from studies undertaken in sample accumulation centers and not from population-based studies. Another reason that prevents the accurate estimation of the incidence of PNS is the difficulty in the diagnosis of PNS. In addition, a universal definition for PNS has not yet been established. Therefore, the incidence of PNS associated with anti-CKB antibody is still unknown. However, in these previous studies, as far as we could find, five out of six PNS patients whose serum was negative for onconeural antibodies were positive for anti-CKB antibodies. It is high frequency, although number of samples is small. On the other hand, anti-CKB antibody was identified from the serum of a gastric cancer patient with paraneoplastic sensorydominant neuropathy, a type of PNS; this finding was reported prior to our studies [6]. Thus, we believe that more onconeural antibodies associated with PNS should be identified to elucidate the molecular mechanism of pathogenesis for PNS and establish a new strategy for the treatment of PNS patients.
The Well-Characterized Onconeural Antibodies
The Paraneoplastic Neurological Syndrome Euronetwork suggests that well-characterized onconeural antibodies (anti-Hu, Yo, Ri, Ma2, amphiphysin, Tr, CV-2, etc.) should be used to definitively diagnose PNS. The definition of a well-characterized antibody is dependent upon the following: (1) identification during routine immunohistochemistry and immunoblotting on recombinant proteins, which must be used to verify their specificities; (2) several reported cases associated with tumors; (3) the description of well-characterized neurological syndromes associated with the antibody; (4) the definite identification of the antibodies in different studies; and (5) the frequency of these antibodies in patients without cancer [7]. The wellcharacterized antibodies can be associated with several different neurologic syndromes, and they are highly predictive of particular cancers (Table 1). This study shows, the well-characterized onconeural antibodies, associated cancers and syndromes. Future studies will possibly allow partially-characterized onconeural antibodies to be upgraded to well-characterized antibodies. The use of other antineuronal antibodies that have been detected occasionally in only one or a few patients with PNS in the diagnosis of PNS is not recommended until sufficient data are obtained.
| Autoantibody | Associated cancer | Neurologic syndromes |
|---|---|---|
| The well-characterized onconeural antibodies | ||
| Anti-Hu | Small cell lung cancer, prostate cancer, lung adenocarcinoma | Encephalomyelitis, paraneoplastic cerebellar degeneration, sensory neuronopathy, limbic encephalitis, etc. |
| Anti-Yo | Gynecologic, breast cancer | Paraneoplastic cerebellar degeneration. |
| Anti-Ri | Breast, gynecologic cancer | Paraneoplastic cerebellar degeneration, opsoclonus myoclonus. |
| Anti-Tr | Hodgkin lymphoma | Paraneoplastic cerebellar degeneration. |
| Anti-CV2 | Small cell lung cancer, lymphoma, breast cancer, lymphoma | Encephalomyelitis, paraneoplastic cerebellar degeneration, sensory neuronopathy, etc. |
| Anti-Ma | Germ-cell tumors of testis, breast cancer | Brainstem encephalitis |
| Anti-Ma2 | Germ-cell tumors of testis, breast cancer | Limbic encephalitis, brainstem encephalitis. |
| Antiamphiphysin | Small cell lung, breast cancer | Stiff-man syndrome, limbic encephalitis. |
Table 1: The well-characterized onconeural antibodies, associated cancers and syndromes.
The Specificity of Anti-CKB Antibody as PNS Onconeural Antibodies
To investigate whether anti-CKB antibodies are specific to PCD patients, we conducted Western blotting and enzyme-linked immunosorbent assay (ELISA) for the detection of anti-CKB antibodies in sera. Analyses using sera from 10 healthy donors and 20 symptomatic cerebellar ataxia patients (two with cerebellitis, 14 with spinocerebellar degeneration, two with cerebral infarction, one with opsoclonus–myoclonus, and one with progressive supranuclear palsy) were performed. Furthermore, an antibody absorption test by recombinant CKB protein was performed for confirming the presence of anti-CKB antibody in serum from the PCD patient. The results indicated that anti-CKB antibodies were not detected in the sera of healthy donors or the ataxia patients [1]. This result clearly showed the target specificity of anti-CKB antibodies as PNS onconeural antibodies and that very important criteria are required for identification [8].
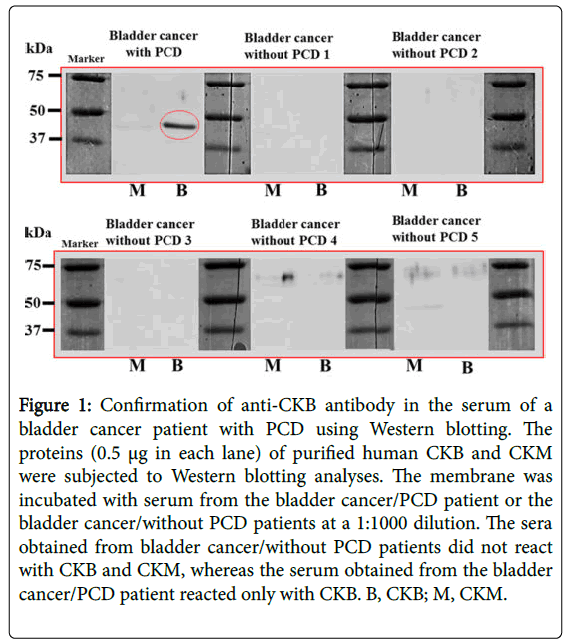
Cancer patients that do not show any symptoms of PCD or PNS do not have these antibodies; thus, well-characterized onconeural antibodies should not simply be a biomarker of cancer. The sera obtained from five bladder cancer patients without PCD did not react with the CKB band (molecular weight, 43 kDa) and creatine kinase muscle-type (CKM), although the serum from the bladder cancer patient with PCD reacted only with CKB by Western blotting (Figure 1) and ELISA [1]. Next, to investigate whether anti-CKB antibodies are specific for PCD patients with small cell lung cancer (SCLC), we performed similar analyses using sera from 10 SCLC patients who did not show any symptoms of PCD or PNS, and the Western blotting results were the same as above (Figure 2) [2]. Therefore, the specificity of anti-CKB antibody as PNS onconeural antibodies was demonstrated. This study shows some recognitions of associated cancers and syndromes by the anti-CKB antibody so far in Table 2 [1,2].
Figure 1: Confirmation of anti-CKB antibody in the serum of a bladder cancer patient with PCD using Western blotting. The proteins (0.5 µg in each lane) of purified human CKB and CKM were subjected to Western blotting analyses. The membrane was incubated with serum from the bladder cancer/PCD patient or the bladder cancer/without PCD patients at a 1:1000 dilution. The sera obtained from bladder cancer/without PCD patients did not react with CKB and CKM, whereas the serum obtained from the bladder cancer/PCD patient reacted only with CKB. B, CKB; M, CKM.
Figure 2: The presence of anti-CKB antibody in the serum of SCLC/PNS patients using Western blotting. Western blotting was performed as described in Figure 1. The membrane was incubated with the serum from the patient or the control serum from a healthy donor at a 1:1000 dilution. The sera from healthy controls did not react with CKB, whereas the sera from the two SCLC/PCD patients reacted only with CKB. B, CKB.
| Autoantibody | Associated cancer | Neurologic syndromes |
|---|---|---|
| The novel onconeural antibody | ||
| Anti-CKB | Urothelial carcinomas of the urinary bladder, small cell lung cancer, hodgkin’s lymphoma, gastric cancer, squamous cell carcinoma of cervical lymph node. | Paraneoplastic cerebellar degeneration, sensory-motor neuropathy, Sensory-dominant neuropathy. |
Table 2: The anti-CKB antibody, associated cancers and syndromes.
CKB is Expressed both in Mouse Cerebellum and Human Cerebellar n Neurons
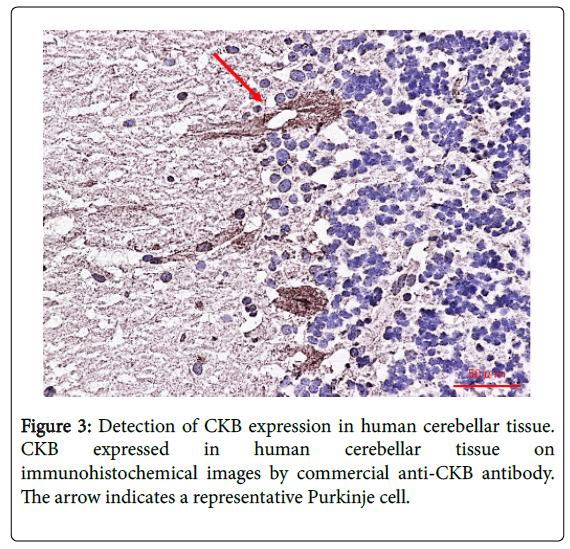
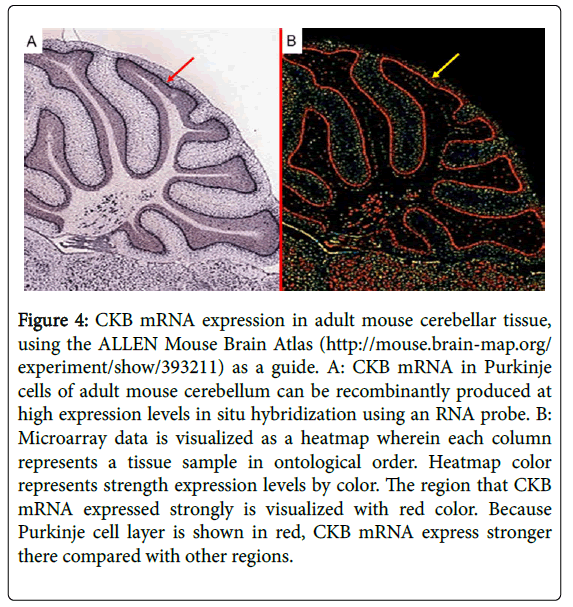
Creatine kinase (CK), a family of enzymes with a highly conserved protein sequence, are vital for maintaining cellular-energy homeostasis. They reversibly catalyze the transfer of phosphate between adenosine triphosphate; ATP and creatine phosphate. There are three cytoplasmic isoenzymes of CK that are readily identified in human tissue. These isoenzymes are dimeric molecules with two dissociable subunits that are designated as CKM or CKB. They can reassociate to form the electrophoretically distinct MM, BB, or MB isotypes. Nearly 100% of the activity results from the MM isoenzyme in skeletal muscle, 5–25% from the MB isoenzyme are in heart muscle, and nearly 100% the BB isoenzyme are in brain and nerve tissue. As CKB is mainly expressed in the brain, neuroprotective effects have been attributed to the administration of creatine. Jost et al. reported that Purkinje cells and glomerular synapses in the granular layer of the cerebellum show a very high level of CKB expression in their soma [9], similar to the results that we obtained [1]. The unique histopathological finding in PCD is the severe loss of Purkinje cells [3]. The speed with which onconeural antibodies enter Purkinje cells and induce Purkinje cell death in vitro may have important implications for human disease. Thus, Purkinje cells are irreversibly and markedly damaged in the early stage of the syndrome, and PCD patients may have symptomatic cerebellar ataxia. Anti-CKB autoantibody in such PCD patients may attack Purkinje cells, deteriorating its functions and activities and causing symptoms of neurological degeneration. Using immunohistochemistry, we previously demonstrated that CKB is expressed in mouse and human cerebellum [1,2] and human cerebellar neurons (Figure 3) [2], and messenger ribonucleic acid (mRNA) of CKB in Purkinje cells of adult mouse cerebellum can be recombinantly produced at high expression levels in situ hybridization using an RNA probe, according to the ALLEN Mouse Brain Atlas (Figure 4). Furthermore, CKB is expressed in Purkinje cells. This result further demonstrates that anti-CKB antibody is associated with PCD in humans.
Figure 4: CKB mRNA expression in adult mouse cerebellar tissue, using the ALLEN Mouse Brain Atlas (http://mouse.brain-map.org/ experiment/show/393211) as a guide. A: CKB mRNA in Purkinje cells of adult mouse cerebellum can be recombinantly produced at high expression levels in situ hybridization using an RNA probe. B: Microarray data is visualized as a heatmap wherein each column represents a tissue sample in ontological order. Heatmap color represents strength expression levels by color. The region that CKB mRNA expressed strongly is visualized with red color. Because Purkinje cell layer is shown in red, CKB mRNA express stronger there compared with other regions.
Expression of CKB in Cancer Specimens
According to the diagnostic criteria for PNS, even if we cannot recognize a tumor, we can definitively diagnose PNS by its characteristic neurological symptoms and the detection of wellcharacterized onconeural antibodies, excluding differential diagnosis [7].
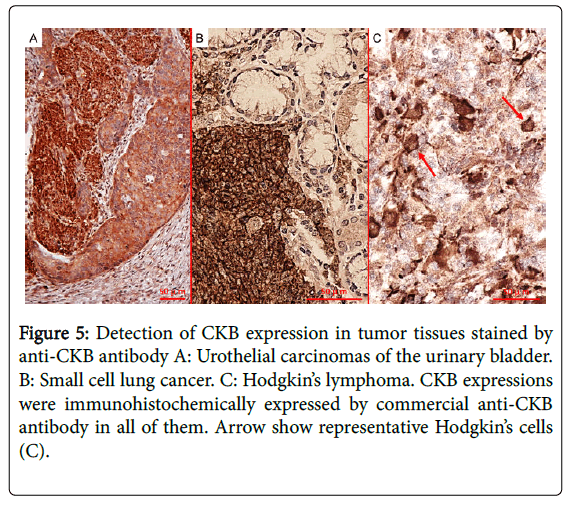
This is because autoantibody profiles observed in patients with PNS imply the targeting of multiple onconeural antigens and predict the patient's neoplasm, but not specific neurological syndromes [10]. However, to detect novel onconeural antibodies for clinical use in the future, proof that the common antigen is expressed both in neurons and in cancer specimens is essential. We also demonstrated CKB expression in cancer specimens (urothelial carcinomas of the urinary bladder, small cell lung cancer, and Hodgkin’s lymphoma) using immunohistochemistry (Figure 5). In addition, it has been reported that the expression of CKB is high in SCLC, breast cancer, prostate cancer, ovarian cancer, and malignant lymphoma [11-17]. Taking into account these findings, we expect that CKB may be a good candidate as an onconeural antigen in many cancers and that PCD associated with anti-CKB antibody may result in some predominant cancers, which is the case with other well-characterized onconeural antibodies, such as anti-Yo and anti-Hu antibodies [7].
Figure 5: Detection of CKB expression in tumor tissues stained by anti-CKB antibody A: Urothelial carcinomas of the urinary bladder. B: Small cell lung cancer. C: Hodgkin’s lymphoma. CKB expressions were immunohistochemically expressed by commercial anti-CKB antibody in all of them. Arrow show representative Hodgkin’s cells (C).
Pathogenesis
The study of the pathogenesis of PCD or PNS caused by intracellular antigens, such as CKB, has not progressed well because it is difficult to show direct evidence that the antibody reaction with intracellular antigens causes PCD or PNS. Till date, various studies have been undertaken to produce an animal model of PCD by passive transfer experiments or active vaccination with an antigen, but they have failed, suggesting the possibility that these antibodies may not be a direct pathogen [18,19] and that the T cell-mediated immune system may be more important for pathogenesis of PCD caused by autoantibodies against intracellular antigens [20]. In vitro studies of T cells derived from patients with ovarian carcinoma and PCD persuasively support the role of onconeural peptide-specific CD8+ T cells as effectors of the inflammatory cytotoxic neuropathology following autoantibodies specific for neural intracellular antigens [21].
Activated CD8+ T cells presumably emigrate from tumor-draining lymph nodes to the systemic circulation, cross the capillary endothelium, enter the central nervous system’s parenchyma, and attack neural cells displaying major histocompatibility complex; MHC class I-bound peptides. However, a recent study showed that Yo antibodies cause Purkinje cell death directly in cerebellar slice cultures [22], and anti-Yo antibodies cause Purkinje cell death by binding to the intracellular 62-kDa Yo antigen [23]. Despite these results, the role of well-characterized onconeural antibodies with regard to the pathogenesis of PNS is unclear.
A conformational epitope is usually a sequence of amino acids composing an antigen that come in direct contact with a receptor of the immune system. An epitope is the part of an antigen that is recognized by specific antibodies. In our previous studies, most serum obtained from controls did not react with CKB and CKM, whereas the serum obtained from the PNS patient reacted only with CKB. A conformational epitope is composed of discontinuous sections of the antigen's amino acid sequence. Thus, amino acid sequences of CKB (human; NCBI: CAA33389.1) and CKM (human; NCBI: AAA52025.1) were compared. The amino acid sequence CKB and CKM is relatively similar in Figure 6. There are little regions of discontinuous sections on the antigen's amino acid sequence. One of the regions may be epitope against anti-CKB antibody. If we utilize this advantage, I think that the epitope against anti-CKB antibody may be likely to be identified earlier than usual.
Closing Remarks
Our studies demonstrated that anti-CKB antibody is produced in some PCD or PNS patients, in whom the condition is associated with a variety of different types of cancers. Therefore, we believe that anti- CKB antibody may be a novel onconeural antibody that is associated with PCD or PNS. Our method of using a proteomic approach was very useful for identifying a new onconeural auto antigen in PCD. I believe that this approach will facilitate detection of new auto antigens for other autoimmune diseases, as well as for PNS. It is necessary to conduct further studies to clarify the pathogenic mechanisms of onconeural antibodies against CKB and determine how they provoke central nerve dysfunction.
I have to admit that this report only states a preliminary result, as there were a limited number of the cases available at the time we executed our experiment. However, I believe that the result shows an intriguing fact; it gives a scientific stand to the historically-screened fact- that is, "the anti-CKB antibody is associated with PNS". In addition, further epidemiological analysis is needed to confirm the presence of an anti-CKB antibody, as well as other well-characterized onconeural antibodies, before this can be considered a useful marker of PNS in the clinical setting.
Acknowledgment
The author would like to thank Enago (www.enago.jp) for the English language review.
Conflict of Interests
The authors declare that they have no conflict of interests.
References
- Tetsuka S, Tominaga K, Ohta E, Kuroiwa K, Sakashita E, et al. (2013)
- Tetsuka S, Yamasawa H, Ikeguchi K, Fujimoto K. (2015)
- Dalmau J, Rosenfeld MR. (2008)
- Giometto B, Grisold W, Vitaliani R, Graus F, Honnorat J, et al. (2010)
- Shams'ili S, Grefkens J, de Leeuw B, van den Bent M, Hooijkaas H, et al. (2003)
- Arawaka S, Daimon M, Sasaki H, Suzuki JI, Kato T. (1998)
- Graus F, Delattre JY, Antoine JC, Dalmau J, Giometto B, et al. (2004)
- Graus F, Saiz A, Dalmau J. (2010)
- Jost CR, Van Der Zee CE, In 't Zandt HJ, Oerlemans F, Verheij M, et al. (2002)
- Pittock SJ, Kryzer TJ, Lennon VA. (2004)
- Carney DN, Zweig MH, Ihde DC, Cohen MH, Makuch RW, et al. (1984)
- Niklinski J, Furman M, Laudanski J, Palynyczko Z, Welk M. (1991)
- Niklinski J, Furman M, Palynyczko Z, Laudanski J, Bulatowicz J. (1991)
- Zarghami N, Yu H, Diamandis EP, Sutherland DJ. (1995)
- Zarghami N, Giai M, Yu H, Roagna R, Ponzone R, et al. (1996)
- Huddleston HG, Wong KK, Welch WR, Berkowitz RS, Mok SC. (2005)
- Ishikawa J, Taniguchi T, Takeshita A, Maekawa M. (2005)
- Graus F, Illa I, Agusti M, Ribalta T, Cruz-Sanchez F, et al. (1991)
- SillevisSmitt PA, Manley G. T, Posner JB. (1995)
- Carpenter EL, Vance BA, Klein RS, Voloschin A, Dalmau J, et al. (2008)
- Albert ML, Darnell JC, Bender A, Francisco LM, Bhardwaj N, et al. (1998)
- Greenlee JE, Clawson SA, Hill KE, Wood BL, Tsunoda I, et al. (2010)
- Greenlee JE, Clawson SA, Hill KE, Wood B, Clardy SL, et al. (2015)
Share This Article
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 14091
- [From(publication date):
December-2016 - Nov 25, 2024] - Breakdown by view type
- HTML page views : 13370
- PDF downloads : 721