Our Group organises 3000+ Global Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ ������ Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
������ Journals gaining more Readers and Citations
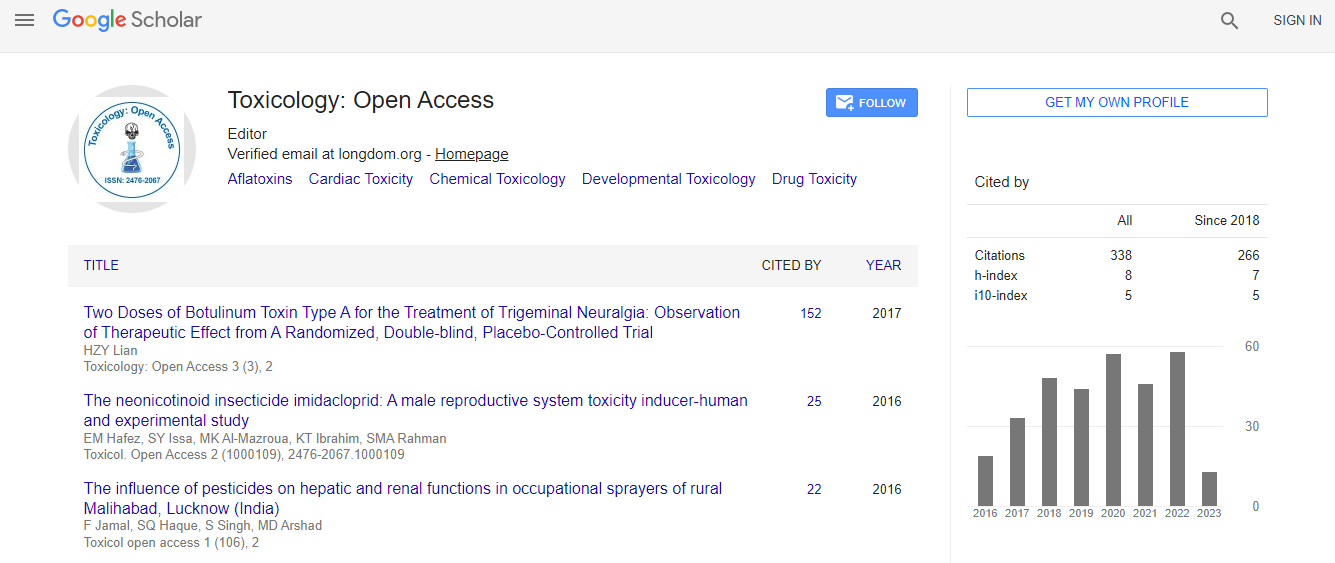
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
Citations : 336
Indexed In
- Google Scholar
- RefSeek
- Hamdard University
- EBSCO A-Z
- Geneva Foundation for Medical Education and Research
- Euro Pub
- ICMJE
Useful Links
Related Subjects
Share This Page
Drug development: Ethics versus efficacy
14th World Congress on Toxicology and Pharmacology
Kumaresh Pandian and Krishnan V
Saveetha Medical College, India
ScientificTracks Abstracts: Toxicol ������
DOI:
Abstract
Objective: To analyze the post-marketing status of molecules approved through the expedited review process in the last quintile. Methods: The observational study was carried out between January 2016 and June 2016. The details of the time taken to approve drugs were collected from the official website on the United States Food and Drug Administration (FDA). The average time taken to review drugs and take a decision following the review was ascertained from the FDA��?s annual release of novel drugs from 2011 to 2015. Information on adverse drug reaction noted after approval was gathered from FDA Drug Safety Communication and FDA Adverse Event Reporting System (FAERS). Results: In the last five years, 166 products were approved by expedited review. Of these 45 (27.1%) did not meet the stringent criteria framed for expedited review. Reports of serious adverse event alerts were submitted for 79 (47.5%) of the 166 molecules. 14 (8.4%) drugs were associated with inducing severe autoimmune disorders. It can be observed that a lower average time of review is positively correlated with a greater number of adverse events (p<0.05) and 37 (45.7%) of the molecules failed to be of any treatment scenario. Conclusion: Drug approval by accelerated review should be stringent. Beneficence and non-maleficence are applicable to the global population and should apply equally to subjects involved in trials. Approving drugs on the basis of trivial evidence is non-scientific and absolutely unethical, since it can lead to clinical failure and produce serious adverse events.Biography
Kumaresh Pandian is currently pursuing his MBBS from Saveetha Medical College, SIMAT. His area or research is drug development under the mentorship of Dr. V Krishnan, Department of Pharmacology, SIMATS in India.
Email:kumaresh_90@ymail.com