Our Group organises 3000+ Global Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ 黑料网 Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
黑料网 Journals gaining more Readers and Citations
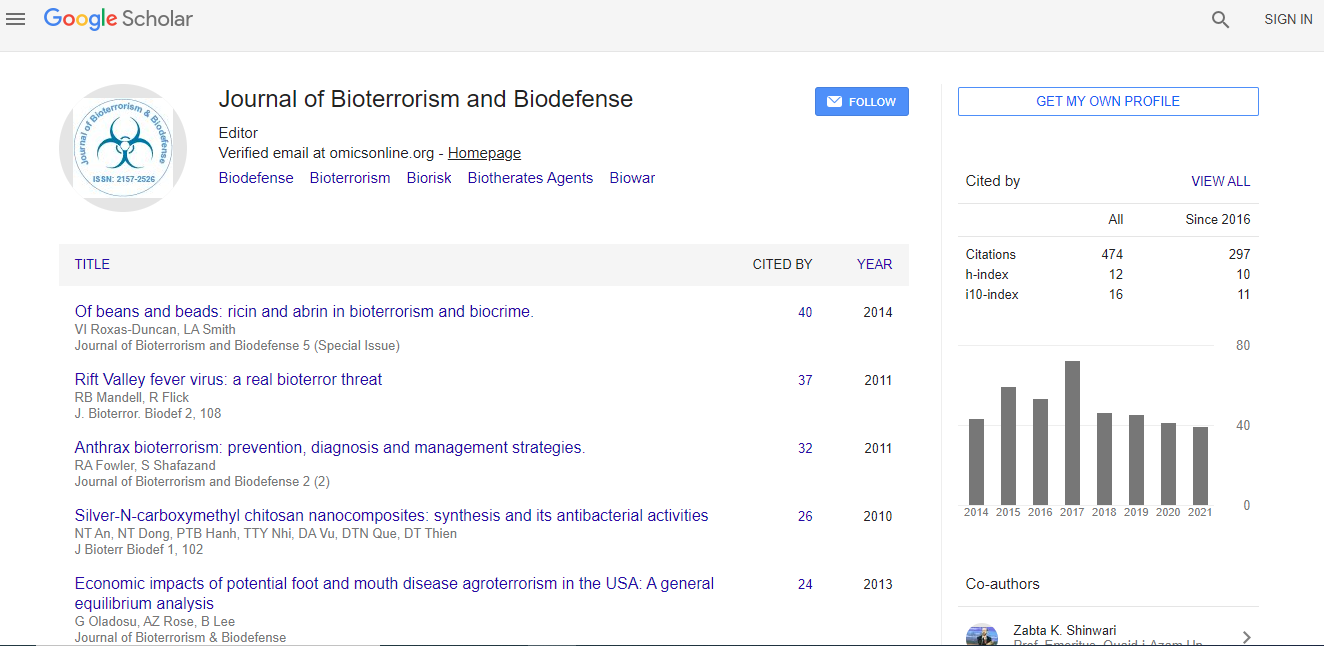
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
Citations : 1129
Indexed In
- CAS Source Index (CASSI)
- Index Copernicus
- Google Scholar
- Sherpa Romeo
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- ResearchBible
- China National Knowledge Infrastructure (CNKI)
- Ulrich's Periodicals Directory
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
Human monoclonal antibodies (mAb): Interrogation of the memory B cell compartment as a powerful platform for the discovery and development of superior therapeutics
International Conference on Biothreats & Biodefense
Aurelio Bonavia
ScientificTracks Abstracts: J Bioterr Biodef
DOI: