Our Group organises 3000+ Global Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ 黑料网 Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
黑料网 Journals gaining more Readers and Citations
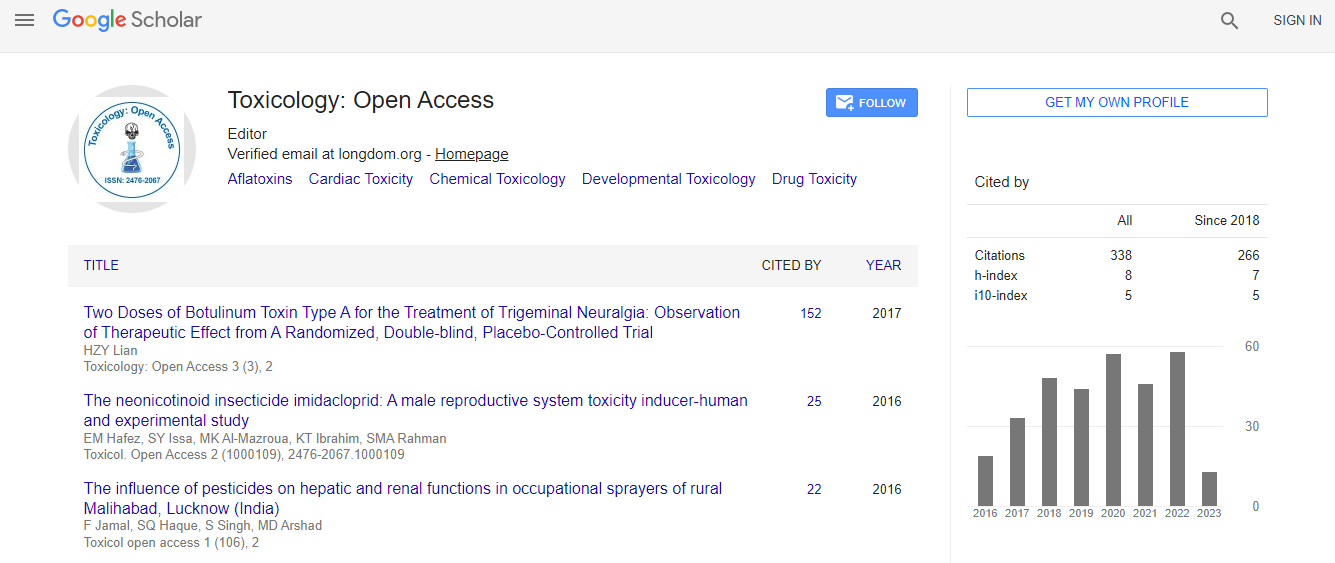
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
Citations : 336
Indexed In
- Google Scholar
- RefSeek
- Hamdard University
- EBSCO A-Z
- Geneva Foundation for Medical Education and Research
- Euro Pub
- ICMJE
Useful Links
Related Subjects
Share This Page
Syntheses and antioxidant activity of novel pyrrol-benzoimidazole derivatives
8th World Congress on Toxicology and Pharmacology
Zeynep Ates-Alagoz, Fikriye Zengin, Rahman Basaran and Benay Can-Eke
Ankara University, Turkey
Posters & Accepted Abstracts: Toxicol 黑料网
DOI:
Abstract
The antioxidants and antioxidant enzyme systems belong to the major protective systems of the organism. Both pyrroles and benzimidazoles exhibit different important biological activities, like antibacterial, antioxidant, cytotoxic properties. For this reason, novel pyrrole-benzimidazole derivatives were designed and synthesized to perform their antioxidant activity. Syntheses of the compounds were carried out starting from commercially available aryl sulfonyl chlorides. Alkylation of the sulfonyl chlorides with iodoethane or iodomethane in the presence of tellurium, rongalite and 1 M aqueous sodium hydroxide gave ethylsulfonyl/methylsulfonyl derivatives. This was followed by reaction with conc. H2SO4 and potassium nitrate to give nitro intermediates. Nucleophilic displacement of the chloro group with several amines in N, N-dimethylformamide and their reduction with hydrogen gas by using palladium carbon and condensation of these derivatives with 1H-pyrrole-2- carbaldehyde gave the targeted pyrrole-benzimidazoles. Purity control and structural elucidation were controlled by using elemental analyzer and H, C-NMR, Mass spectrometers, respectively. Their in vitro effects on rat liver microsomal NADPHdependent lipid peroxidation (LP) levels and ethoxyresorufin O-deethylase (EROD) activity were determined. All synthesized compounds showed moderate activity on LP levels when compared with BHT. Compounds 1-7 displayed strong inhibitory activity on LP and inhibition rate was 77-65%. However, no significant inhibitory effect was obtained on EROD activity.Biography
Zeynep Ates-Alagoz usually performs the organic synthesis work. Her research interests are in the area of drug discovery focusing on both organic synthesis and structure-activity relationships. She has synthesized novel compounds having indole/benzimidazole/thiazolidinedione ring systems and has evaluated their antioxidant, antimicrobial and anticancer activities. She has also conducted structure-activity studies of retinoidal and melatonergic compounds. Currently, she is working on several projects including syntheses of novel NMDA receptor antagonists for treatment of Alzheimer’s disease, and syntheses of novel radiosensitizers for anticancer activity and small molecules for antioxidant activity.
Email: zates@pharmacy.ankara.edu.tr