Our Group organises 3000+ Global Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ ║┌┴Ž═° Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
║┌┴Ž═° Journals gaining more Readers and Citations
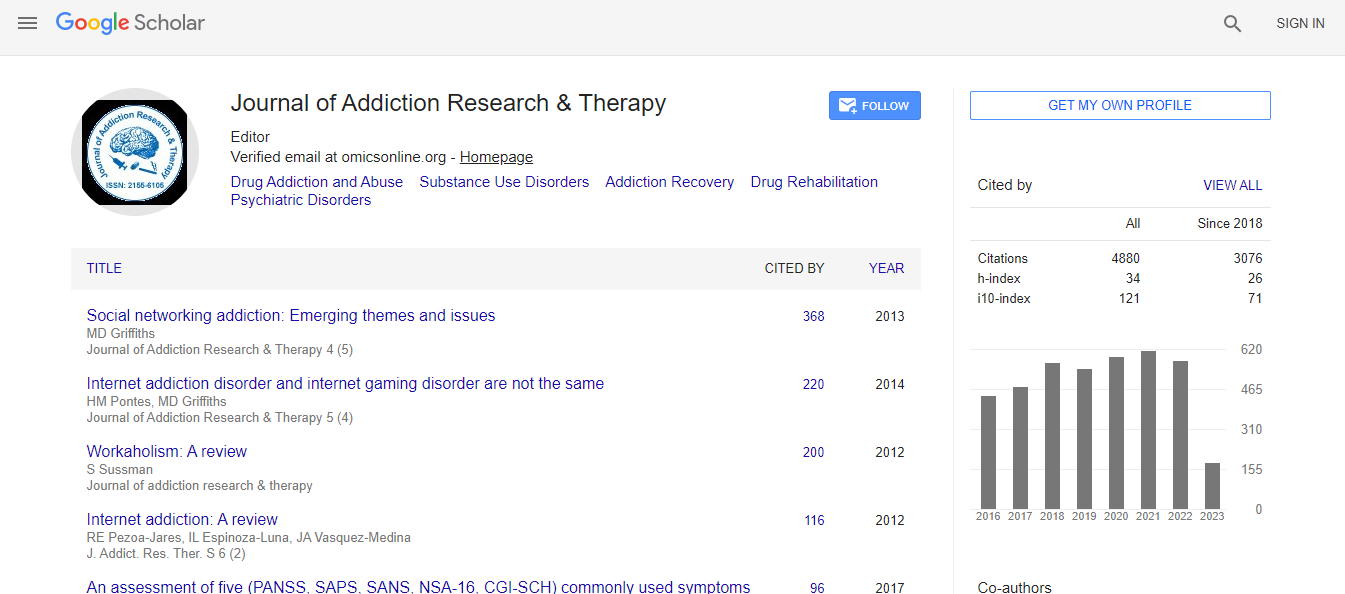
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
Citations : 4859
Indexed In
- CAS Source Index (CASSI)
- Index Copernicus
- Google Scholar
- Sherpa Romeo
- Open J Gate
- Genamics JournalSeek
- Academic Keys
- JournalTOCs
- SafetyLit
- China National Knowledge Infrastructure (CNKI)
- Electronic Journals Library
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- SWB online catalog
- Virtual Library of Biology (vifabio)
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
Accumulation of highly stable ├?┬?FosB-Isoforms and its targets inside the reward system of chronic drug abusers
6th World Congress on Addiction Disorder & Addiction Therapy
Monika Heidemarie Seltenhammer
Medical University of Vienna, Austria
ScientificTracks Abstracts: J Addict Res Ther
DOI:
Abstract
Background: The ~33kD transcription factor ├?┬?FosB, a Fos-family protein and belonging to the immediate early genes (IEGs), is initiated in the acute phase as a response to a wide range of effects such as drugs, stress, and several external stimuli. ├?┬?FosB forms heterodimers with Jun proteins to generate active activator protein-1 (AP-1) complexes. They bind to AP-1 sites in the promoter regions of many neural genes. To date, several downstream target genes for ├?┬?FosB have been identified being involved in molecular pathways concerning addictive behavior, memory and learning. In answer to chronic stimuli, the rather unstable ~33kD transcription factor ├?┬?FosB is replaced by robust ~35-37 kD isoforms due to epigenetic splicing and different phosphorylation steps. The result is that these highly stable isoforms accumulate in the nucleus accumbens (NAc), a structure close to the hippocampus (HPC), playing a key role within the reward center of the brain. These stabilized ~35-37 kD ├?┬?FosB derivatives linger in the brain for several weeks or longer even though the chronic stimulus has been removed ├ó┬?┬? a fact that seems to be responsible for the development of sustained neuronal plasticity, (drug associated) long-term potentiation (LTP) and memory. In case of chronic drug abuse, the end result is addictive behavior and may be a crucial factor for high relapse rates. Research Questions: Is it possible to detect these highly stable ├?┬?FosB isoforms in post-mortem brain-tissue samples of chronic drug abusers? Can this accumulation also be regarded as source of dependence-memory and high relapse rates? Methods: ├?┬?FosB and cAMP response element binding protein (CREB), brain derived neurotrophic factor (BDNF), JunD, nuclear factor kappa B (NF├?┬║B), and cyclin-dependent kinase 5 (Cdk5) in both of the NAc and HPC of deceased chronic human opioid addicts were proven by immunohistochemistry even with a prolonged postmortem interval (PMI) of 8.47├?┬▒2.61 days. Moreover accumulated ~35-37 kD DFosB isoforms could be detected in the NAc of the same samples by immunoblotting. Results: All determined proteins showed a significant increased staining pattern in brain samples of chronic drug abusers in comparison non-drug users (p<0.05) according to Wilcoxon-Two-Sample Test. Further, accumulated ~35-37 kD ├?┬?FosB isoforms were detectable in NAc samples of long-term drug addicts by immunoblotting in contrast to the control group, where no trace of any isoform was verifiable (p<0.05) according to Wilcoxon-Two-Sample Test. Key Conclusions: Taken together with the results of already published functional in-vivo animal experiments, our findings provide additional evidence of the potential strong impact of ├?┬?FosB on its downstream transcriptional targets, which are in turn responsible for sustainable effects and serious adaptations in the brain that lead to addictive behavior and dependence memory.Biography
Monika Heidemarie Seltenhammer completed her VMD and PhD from VMU in Austria and Post-doctoral studies from Veterinary University of Vienna, Max Perutz Laboratories and Medical University of Vienna in Austria, where her core area of scientific work mainly comprised of cancer research (melanoma) and pathology, but also immunology, neurology and virology. She has received several honor and awards. She is a Leading Member of the Scientific Staff of Dr. Daniele Ugo Risser at the Department of Forensic Medicine of the Medical University Vienna, where she specializes in Neurobiology and Addiction Behavior.