Our Group organises 3000+ Global Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ ������ Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
������ Journals gaining more Readers and Citations
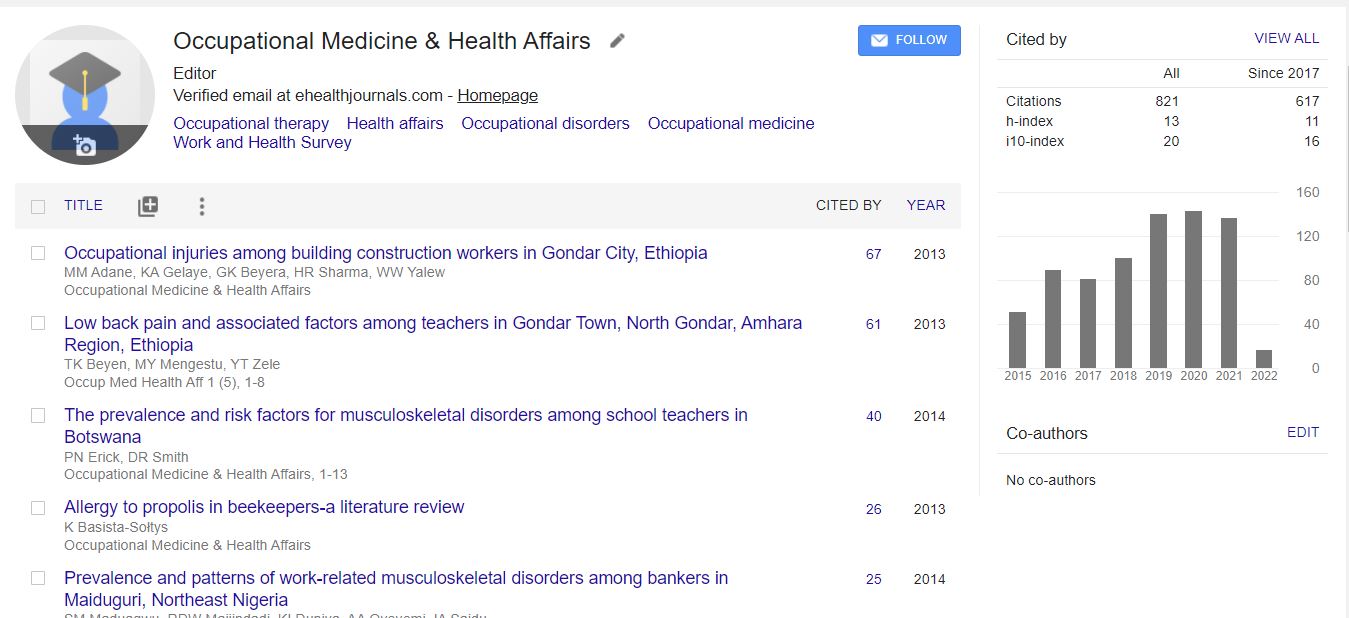
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
Citations : 1907
Indexed In
- Index Copernicus
- Google Scholar
- Open J Gate
- Academic Keys
- China National Knowledge Infrastructure (CNKI)
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- Geneva Foundation for Medical Education and Research
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
ENHANCEMENT OF OXYGEN FUNCTIONAL GROUPS ON OLIVE STONES ACTIVATED CARBON SURFACE TO IMPROVE HEAVY METAL REMOVAL FROM SINGLE AND BINARY AQUEOUS SYSTEMS
International Conference on Environmental Health & Safety
Thouraya Bohli and Abdelmottaleb Ouederni
University of Gab�?¨s, Tunisia
ScientificTracks Abstracts: Occup Med Health Aff
DOI:
Abstract
Heavy metals are common pollutant found in various industrial effluents. The stricter environmental regulations on the discharge of heavy metals make it necessary to develop various technologies for their removal. Adsorption on activated carbon was considered to be the more effective process especially at low concentrations. The surface characteristics and chemical properties of activated carbon are the most important factors that determine the adsorption capacity. These two factors can be changed through certain surface oxidizing methods of activated carbon. In this work, an activated carbon prepared from olive stones by chemical way using phosphoric acid (COSAC) was further undergoes treatments with nitric acid, ozone, CuO impregnation and Al2O3 impregnation to improve the surface chemistry. Activated carbons were characterized by BET, FTIR and Boehm titration. Treated ACs show a decrease in both specific surface area and micro pore volume, and lead to a fixation of high amounts of oxygen functional groups, especially when nitric acid and ozone were used, thus making the carbon surfaces more hydrophilic. Prepared ACs were used as an adsorbent matrix for Co(II), Ni(II) and Cu(II) metal ions removal from aqueous solution. Adsorption isotherms obtained at 30�?°C show that the modified ACs are able to sorb more Co(II), Cu(II) and Ni(II) from aqueous solution. Nitric acid treated AC was found to be the most favourable one since higher heavy metal ions uptake are observed when using this material. COSAC and nitric acid-treated olive stones activated carbon were tested and compared in their ability to remove Metal ions from binary systems and results show synergies, inhibitor and enhancement effects and higher adsorbed amounts as compared to single systems.Biography
Email: bohlithouraya@gmail.com