Our Group organises 3000+ Global Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ ������ Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
������ Journals gaining more Readers and Citations
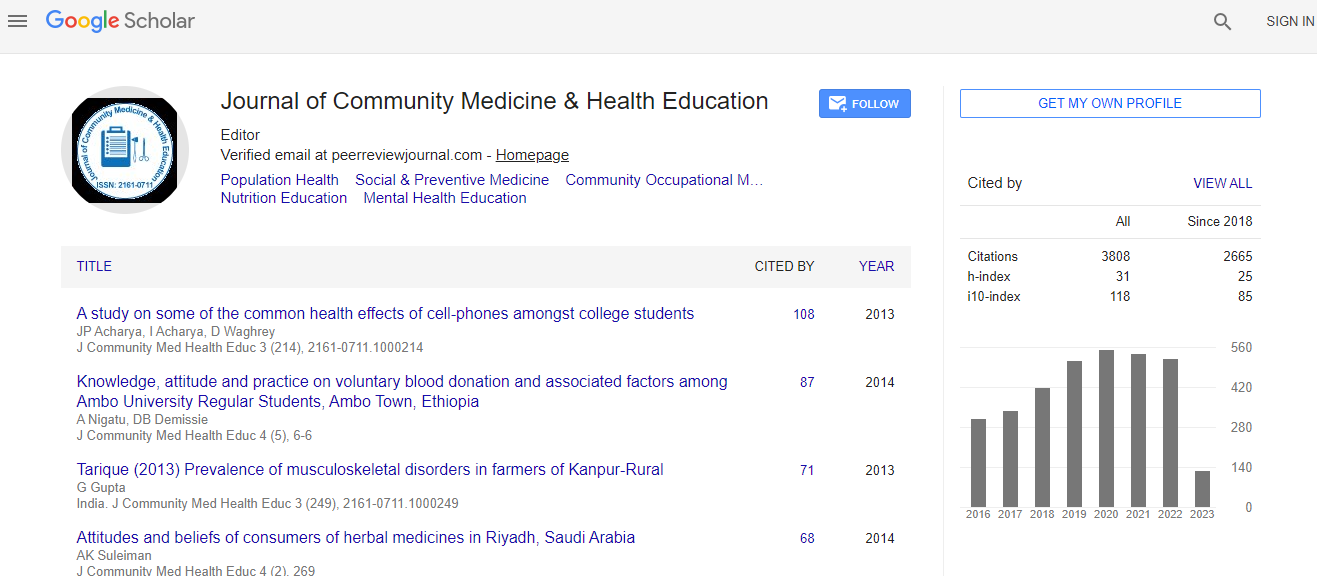
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
Citations : 5447
Indexed In
- Index Copernicus
- Google Scholar
- Sherpa Romeo
- Genamics JournalSeek
- SafetyLit
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
Methods for including patient preferences into the risk and benefit decisions of the Food and Drug Administration for drugs and devices: The input of behavioral economics
Joint Event on 3rd World Congress on Medical Sociology & Public Health & International Conference on Public health and Epidemic diseases
Leslie Wilson
University of California, USA
Keynote: J Community Med Health Educ
DOI:
Abstract
Problem Statement: The Food and Drug Administration has historically made approval decisions for new devices without meaningful patient input. Individual patients experience effects of diseases and therapies differently and their trade-offs between therapy risks and benefits may differ from their healthcare provider or FDA. In 2016 an FDA guidance was released to include patient preference information into device submissions. Behavioral economics and utility theory offer multiple methods to measure patients’ preferences for risks and benefits of treatments, yet FDA is uncertain which methods are best for approval decisions. This study purpose is to describe the current status of patient preference measurement in FDA and present our approach to development and validating different conceptual measurement methods for new prosthetic devices for patients with limb loss. Methodology and Theoretical Orientation: A modified meta-ethnographic approach, including concept synthesis, interviews and pilot testing were used to develop 2 methodologically different patient preference measures; choice-based conjoint (CBC) and a standard gamble (SG) utility method. We describe development and compare responses of the three different approaches in 20 subjects with upper limb loss. Findings: There was variability across our subjects in reason and level of limb loss, age, time with limb loss and prosthetic experience. The method of attribute selection led to the need for video use to better describe the beneficial motion of new prosthetics. Patients showed a stronger preference for favoring benefits and ignoring risks when preferences were measured using video, but all methods demonstrated consistency in preferences across attributes of risk and benefit. Conclusion and Significance: Persons with upper limb loss were able to make trade-offs in decisions using risks and benefits of two new innovations in prosthetic devices. The FDA will be able to use our results validating the best methods to use, to update their guidance on patient preference methods.Biography
Leslie Wilson, PhD, is a professor at University of California, San Francisco. Her expertise is in Health Economics and Health Policy, including cost-effectiveness, cost benefit and costing and economics of different models of health care delivery and methods of measuring patient preference using behavioral economics and utility concepts. She currently works with the FDA to better validate methods for measuring patient preference for input into FDA device approvals. She has multiple years of experience teaching decision analysis and patient preference methods and has multiple publications testing how patients weigh risks and benefits of treatments. The current work on validating patient preference methods is funded by a Burroughs Welcome Fund grant and funding from the UCSF CERSI center. This work will provide usable input into the FDA guidance documents on patient preference.
E-mail: Leslie.Wilson@ucsf.edu