Our Group organises 3000+ Global Events every year across USA, Europe & Asia with support from 1000 more scientific Societies and Publishes 700+ ║┌┴¤═° Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
║┌┴¤═° Journals gaining more Readers and Citations
700 Journals and 15,000,000 Readers Each Journal is getting 25,000+ Readers
Recommended Conferences
Toronto, Canada
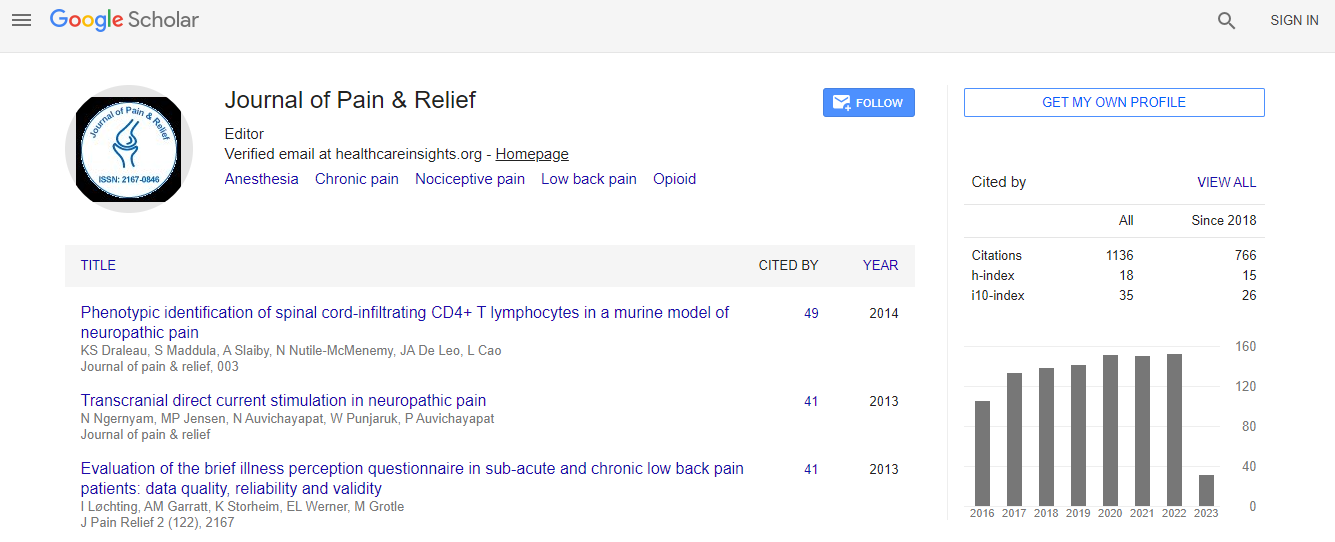
Citations : 1583
Indexed In
- Index Copernicus
- Google Scholar
- Open J Gate
- Genamics JournalSeek
- Cosmos IF
- RefSeek
- Hamdard University
- EBSCO A-Z
- OCLC- WorldCat
- Publons
- Geneva Foundation for Medical Education and Research
- Euro Pub
- ICMJE
Useful Links
Recommended Journals
Related Subjects
Share This Page
How to report statistics in medicine?
4th International Conference on Pain Medicine
Michelle Secic
Secic Statistical Consulting, Inc., USA
ScientificTracks Abstracts: J Pain Relief
DOI:
Abstract
I suspect you do not have the time or desire to learn all the nuances, formulas and theories in statistical computations. You just want to know what tests/methods to use for your study and what needs to be reported. Whether you are reporting results to the FDA or in the medical literature or to upper management, etc. you will need to ensure you are reporting your results accurately, for your type of study. You can think of my guidelines as Cliff├ó┬?┬?s notes for reporting statistics in medicine. This is just a small snapshot of the comprehensive guide. I will discuss the following three common study objectives: 1) group comparison, 2) performance goal and 3) identify risk factors. For each of the three common study objectives, I will first present examples accurately stating the objectives. Second, I will provide a comprehensive template for reporting the results from each of the three types of studies. The templates will include relevant medical examples, numeric results, statistical findings, tests/methods, etc. Finally, I will provide the full list of concepts covered in my guidelines.Biography
Michelle Secic has over 25 years of biostatistical experience. She has worked as a Biostatistician at the Cleveland Clinic Foundation (CCF) for 11 years and was Manager of the Research Section of the Transplant Center at CCF. She then became President of Secic Statistical Consulting, Inc. where she collaborates with researchers from hospitals, pharmaceutical companies, medical device companies and CROs around the world. She coauthored the book, ‘How to Report Statistics in Medicine: Annotated Guidelines for Authors, Editors and Reviewers’ which was published by the American college of physicians (1st edition 1997, translation to Chinese 2002, 2nd edition 2006, translation into Japanese 2010, translation into Russian 2013). This book is referenced by the FDA in their guidance for industry document, statistical guidance on reporting results from studies evaluating diagnostic tests..